Diquat
 | |
 | |
| Names | |
|---|---|
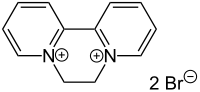
| IUPAC name
6,7-Dihydrodipyrido[1,2-a:2',1'-c]pyrazinediium dibromide | |
| Other names
1,1'-Ethylene-2,2'-bipyridyldiylium dibromide | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.001.436 |
| KEGG | |
| PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C12H12Br2N2 | |
| Molar mass | 344.05 g·mol−1 |
| Appearance | Yellow crystals[1] |
| Density | 1.22 - 1.27 g/cm3 |
| Melting point | 335 °C (635 °F; 608 K) |
| Boiling point | Decomposes[1] |
| 70% (20°C)[1] | |
| Vapor pressure | <0.00001 mmHg (20°C)[1] |
| Hazards | |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
none[1] |
| REL (Recommended) |
TWA 0.5 mg/m3[1] |
| IDLH (Immediate danger) |
N.D.[1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Diquat is a contact herbicide that produces desiccation and defoliation most often available as the dibromide, diquat dibromide.[2] Brand names for this formulation include Aquacide, Dextrone, Preeglone, Deiquat, Spectracide, Detrone, Reglone, Reglon, Reglox, Tribune, Ortho-Diquat, Weedtrine-D,[3] Weedol 2 and, in combination with glyphosate, Resolva.[4]
Diquat is a non-selective herbicide that acts quickly to damage only those parts of the plant to which it is applied.[5] It has been used in pre-harvest crop desiccation.[6] It bonds strongly to mineral and organic particles in soil and water where it remains without significant degradation for years. However, bound to clays diquat is biologically inactive at concentrations typically observed in agricultural soils.[5]
Diquat dibromide is moderately toxic. It may be fatal to humans if swallowed, inhaled, or absorbed through the skin in large quantities.[5]
Production
Pyridine is oxidatively coupled to 2,2'-bipyridine over a heated Raney nickel catalyst. The ethylene bridge is formed by the reaction with 1,2-dibromoethane:[7]
See also
References
- 1 2 3 4 5 6 7 "NIOSH Pocket Guide to Chemical Hazards #0243". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Merck Index, 11th Edition, 3359.
- ↑ PubChem listing for diquat dibromide
- ↑ Weedkillers for Home Gardeners. RHS Advisory Service, Royal Horticultural Society, February 2013. Accessed May 2013.
- 1 2 3 EXTOXNET, Pesticide Information Project
- ↑ "The agronomic benefits of glyphosate in Europe" (PDF). Monsanto Europe SA. February 2010. Retrieved June 2, 2013.
- ↑ "Paraquat and Diquat". IPCS INCHEM.
