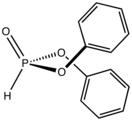
Diphenylphosphite
 | |
| Names | |
|---|---|
| Other names
Phosphonic acid, diphenyl ester | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChemSpider | |
| PubChem CID |
|
| |
| |
| Properties | |
| C12H11O3P | |
| Molar mass | 234.19 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.2268 g/cm3 |
| Melting point | 12 °C (54 °F; 285 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Diphenylphosphite is an organophosphorus compound with the formula (C6H5O)2P(O)H. The molecule is tetrahedral. It is a colorless viscous liquid. The compounds can be prepared by treating phosphorus trichloride with phenol. Many analogues can be prepared similarly. One illustrative reaction, diphenylphosphite, aldehydes, and amines react to afford α-aminomethylphosphites (aminophosphonates).[1]
References
- ↑ Bhagat, Srikant; Chakraborti, Asit K. (2007). "An Extremely Efficient Three-Component Reaction of Aldehydes/Ketones, Amines, and Phosphites (Kabachnik-Fields reaction) for the Synthesis of α-Aminophosphonates Catalyzed by Magnesium Perchlorate". Journal of Organic Chemistry. 72: 1263–1270. doi:10.1021/jo062140i.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.