Diphenylketene
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2,2-Di(phenyl)ethenone | |
| Other names
Diphenylethenone | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChemSpider | |
| PubChem CID |
|
| |
| |
| Properties | |
| C14H10O | |
| Molar mass | 194.23 g·mol−1 |
| Appearance | Red-orange oil |
| Melting point | 8 to 9 °C (46 to 48 °F; 281 to 282 K) |
| Boiling point | 118 to 120 at 1mmHg |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Diphenylketene is a chemical substance of the ketene family. Diphenylketene, like most disubstituted ketenes, is a red-orange oil at room temperature and pressure. Due to the successive double bonds in the ketene structure R1R2C=C=O, diphenyl ketene is a heterocumule. The most important reaction of diphenyl ketene is the [2+2] cycloaddition at C-C, C-N, C-O, and C-S multiple bonds.[1]
History
Diphenyl ketene was first isolated by Hermann Staudinger in 1905 and identified as the first example of the exceptionally reactive class of ketenes[2] with the general formula R1R2C=C=O (R1=R2=phenyl group).[3]
Preparation
The first synthesis by H. Staudinger was based on 2-chlorodiphenylacetyl chloride (prepared from hydroxy(diphenyl)acetic acid and thionyl chloride[4]) from which two chlorine atoms are cleaved with zinc in a dehalogenation reaction:[2]

An early synthesis uses benzilmonohydrazone (from Diphenylethanedione and hydrazine hydrate[5]), which is oxidized with mercury(II)oxide and calcium sulfate to form mono-diazoketone, and is then converted into the diphenylketene at 100 °C under nitrogen elimination in 58% yield:[6]

A further early diphenylketene synthesis originates from Eduard Wedekind, who had already obtained diphenyl ketene in 1901 by the dehydrohalogenation of diphenylacetyl chloride with triethylamine, without isolation and characterization though.[7] This variant was also described in 1911 by H. Staudinger.[8]
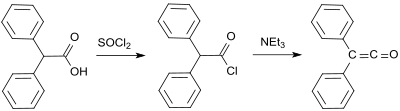
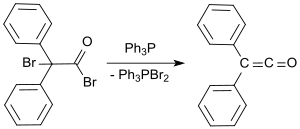
A standard laboratory protocol[9] is based on the Staudinger method and yields diphenyl ketene as an orange oil in yields of 53 to 57%.[10] In a more recent process, 2-bromo-2,2-diphenylacetyl bromide is reacted with triphenylphosphine to give diphenyl ketene in yields up to 81%.[11]
Recently, a synthesis of diphenyl ketene from diphenylacetic acid and the Hendrickson reagent (triphenylphosphonium anhydride-trifluoromethanesulfonate)[12] with water elimination in 72% yield has been reported.[13]

Properties
Diphenyl ketene is at room temperature an orange-colored to red oil (with the color of concentrated potassium dichromate solution[2]) which is miscible with nonpolar organic solvents (such as diethyl ether, acetone, benzene, tetrahydrofuran, chloroform)[14] and solidifies in the cold forming yellow crystals.[2] The compound is easily oxidized by air but can be stored in tightly closed containers at 0 °C for several weeks without decomposition[9] or in a nitrogen atmosphere with the addition of a small amount of hydroquinone as a polymerization inhibitor[6].
Reactivity
Diphenylketene can undergo attack from a host of nucleophiles, including alcohols, amines, and enolates with fairly slow rates. These rates can be increased in the presence of catalysts. At present the mechanism of attack is unknown, but work is underway to determine the exact mechanism.
References
- ↑ H. Ulrich (1967), Cycloaddition Reactions of Heterocumulenes, New York: Academic Press, p. 374
- 1 2 3 4 H. Staudinger (1905), "Ketene, eine neue Körperklasse" (in German), Ber. Dtsch. Chem. Ges. 38 (2): pp. 1735–1739, doi:10.1002/cber.19050380283
- ↑ T.T. Tidwell (2005), "The first century of ketenes (1905–2005): The birth of a versatile family of reactive intermediates" (in German), Angew. Chem. 44 (36): pp. 5778–5785, doi:10.1002/anie.200500098
- ↑ F.E. King, D. Holmes (1947), "Synthetic mydriatics. Diphenylchloroacetyl chloride as a reagent for the preparation of benzylic esters of tertiary amino-alcohols", J. Chem. Soc.: pp. 164–168, doi:10.1039/JR9470000164
- ↑ T. Curtius, K. Thun (1891), "Einwirkung von Hydrazinhydrat auf Monoketone und Orthodiketone", J. Prakt. Chem. 44 (2): pp. 161–186, doi:10.1002/prac.18910440121
- 1 2 Org. Synth. doi:10.15227/orgsyn.020.0047. Missing or empty
|title=(help) - ↑ E. Wedekind (1901), "Ueber die Gewinnung von Säureanhydriden mit Hülfe von tertiären Aminen", Ber. Dtsch. Chem. Ges. 34 (2): pp. 2070–2077, doi:10.1002/cber.190103402122
- ↑ H. Staudinger (1911), "Über Ketene.XIX. Über Bildung und Darstellung des Diphenylketens", Ber. Dtsch. Chem. Ges. 44 (2): pp. 1619–1623, doi:10.1002/cber.19110440258
- 1 2 Org. Synth. doi:10.15227/orgsyn.052.0036. Missing or empty
|title=(help) - ↑ Submitted by Edward C. Taylor Alexander McKillop, and George H. Hawks. Checked by C. J. Michejda, D. D. von Riesen, R. W. Comnick, and Henry E. Baumgarten. (1972). "DIPHENYLKETENE [Ethenone, diphenyl-]". Organic Syntheses. 52: 36. doi:10.15227/orgsyn.052.0036.
- ↑ S.D. Darling, R.L. Kidwell (1968), "Diphenylketene. Triphenylphosphine dehalogenation of .alpha.-bromodiphenylacetyl bromide" (in German), J. Org. Chem. 33 (10): pp. 3974–3975, doi:10.1021/jo01274a074
- ↑ J.I. McCauley (2012), "Hendrickson reagent (triphenylphosphonium anhydride trifluormethane sulfonate", Synlett 23 (20): pp. 2999–3000, doi:10.1055/s-0032-1317486
- ↑ Z. Moussa (2012), [arkat-usa.org "The Hendrickson ‘POP’ reagent and analogues thereof: synthesis, structure, and application in organic synthesis"], ARKIVOC i: pp. 432–490, arkat-usa.org
- ↑ J.W. Leahy (2001), "Diphenylketene", e-EROS Encyclopedia of Reagents for Organic Synthesis, doi:10.1002/047084289X.rd421