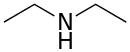
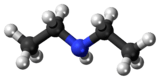
Diethylamine
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
N-Ethylethanamine | |
| Other names
(Diethyl)amine Diethylamine (deprecated[2]) | |
| Identifiers | |
| 3D model (JSmol) |
|
| 605268 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.003.380 |
| EC Number | 203-716-3 |
| MeSH | diethylamine |
| PubChem CID |
|
| RTECS number | HZ8750000 |
| UNII | |
| UN number | 1154 |
| |
| |
| Properties | |
| C4H11N | |
| Molar mass | 73.14 g·mol−1 |
| Appearance | Colourless liquid |
| Odor | fishy, ammoniacal |
| Density | 0.7074 g mL−1 |
| Melting point | −49.80 °C; −57.64 °F; 223.35 K |
| Boiling point | 54.8 to 56.4 °C; 130.5 to 133.4 °F; 327.9 to 329.5 K |
| Miscible | |
| log P | 0.657 |
| Vapor pressure | 24.2–97.5 kPa |
| Henry's law constant (kH) |
150 μmol Pa−1 kg−1 |
| -56.8·10−6 cm3/mol | |
| Refractive index (nD) |
1.385 |
| Thermochemistry | |
| 178.1 J K−1 mol−1 | |
| Std enthalpy of formation (ΔfH |
−131 kJ mol−1 |
| Std enthalpy of combustion (ΔcH |
−3.035 MJ mol−1 |
| Hazards | |
| Safety data sheet | hazard.com |
| GHS pictograms |    |
| GHS signal word | DANGER |
| H225, H302, H312, H314, H332 | |
| P210, P280, P305+351+338, P310 | |
| NFPA 704 | |
| Flash point | −23 °C (−9 °F; 250 K) |
| 312 °C (594 °F; 585 K) | |
| Explosive limits | 1.8–10.1% |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
540 mg/kg (rat, oral) 500 mg/kg (mouse, oral)[3] |
| LC50 (median concentration) |
4000 ppm (rat, 4 hr)[3] |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
TWA 25 ppm (75 mg/m3)[4] |
| REL (Recommended) |
TWA 10 ppm (30 mg/m3) ST 25 ppm (75 mg/m3)[4] |
| IDLH (Immediate danger) |
200 ppm[4] |
| Related compounds | |
| Related amines |
|
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Diethylamine is an organic compound with the formula (CH3CH2)2NH. It is a secondary amine. It is a flammable, weakly alkaline liquid that is miscible with most solvents. It is a colorless liquid, but commercial samples often appear brown due to impurities. It has a strong ammonia-like odor.
Production and uses
Diethylamine is manufactured by the alumina-catalyzed reaction of ethanol and ammonia. It is obtained together with ethylamine and triethylamine. Annual production of three ethylamines was estimated in 2000 to be 80,000,000 kg.[5]
It is used in the production of corrosion inhibitor N,N-diethylaminoethanol, by reaction with ethylene oxide. It is also a precursor to a wide variety of other commercial products. Diethylamine can be used in the production of LSD and therefore it is strictly monitored in the U.S.
Safety
Diethylamine has low toxicity, but the vapor causes transient impairment of vision.[5]
References
- ↑ Merck Index, 12th Edition, 3160
- ↑ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 671. ISBN 978-0-85404-182-4. doi:10.1039/9781849733069-FP001.
- 1 2 "Diethylamine". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- 1 2 3 "NIOSH Pocket Guide to Chemical Hazards #0209". National Institute for Occupational Safety and Health (NIOSH).
- 1 2 Karsten Eller, Erhard Henkes, Roland Rossbacher, Hartmut Höke "Amines, Aliphatic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. doi:10.1002/14356007.a02_001
