Diatom
| Diatoms | |
|---|---|
 | |
| Marine diatoms | |
| Scientific classification | |
| Domain: | Eukaryota |
| (unranked): | SAR |
| Superphylum: | Heterokonta |
| Phylum: | Ochrophyta |
| Class: | Bacillariophyceae Dangeard, 1933[1] |
| Synonyms | |
| |
Diatoms[6] are a major group of algae, and are among the most common types of phytoplankton. Diatoms are unicellular, although they can form colonies in the shape of filaments or ribbons (e.g. Fragilaria), fans (e.g. Meridion), zigzags (e.g. Tabellaria), or stars (e.g. Asterionella). The first diatom formally described in scientific literature, the colonial Bacillaria paradoxa, was found in 1783 by Danish naturalist Otto Friedrich Müller. Diatoms are producers within the food chain. A unique feature of diatom cells is that they are enclosed within a cell wall made of silica (hydrated silicon dioxide) called a frustule.[7] These frustules show a wide diversity in form, but are usually almost bilaterally symmetrical, hence the group name. The symmetry is not perfect since one of the valves is slightly larger than the other, allowing one valve to fit inside the edge of the other. Fossil evidence suggests that they originated during, or before, the early Jurassic period. Only male gametes of centric diatoms are capable of movement by means of flagella. Diatom communities are a popular tool for monitoring environmental conditions, past and present, and are commonly used in studies of water quality.
General biology

More than 200 genera of living diatoms are known, with an estimated 100,000 extant species.[8][9][10][11] Diatoms are a widespread group and can be found in the oceans, in fresh water, in soils, and on damp surfaces. They are one of the dominant components of phytoplankton in nutrient-rich coastal waters and during oceanic spring blooms since they can divide more rapidly than other groups of phytoplankton.[12] Most live pelagically in open water, although some live as surface films at the water-sediment interface (benthic), or even under damp atmospheric conditions. They are especially important in oceans, where they contribute an estimated 45% of the total oceanic primary production of organic material.[13] Spatial distribution of marine phytoplankton species is restricted both horizontally and vertically.[14][15] Though most are microscopic, some species of diatoms can reach up to 2 mm in length.


Diatoms belong to a large group called the heterokonts, including both autotrophs (e.g., golden algae, kelp) and heterotrophs (e.g., water moulds). Their yellowish-brown chloroplasts are typical of heterokonts, having four membranes and containing pigments such as the carotenoid fucoxanthin. Individuals usually lack flagella, but they are present in male gametes of the centric diatoms and have the usual heterokont structure, except they lack the hairs (mastigonemes) characteristic in other groups. Most diatoms are nonmotile, as their relatively dense cell walls cause them to readily sink. Planktonic forms in open water usually rely on turbulent mixing of the upper layers by the wind to keep them suspended in sunlit surface waters. It was once assumed that species actively regulate their buoyancy via intracellular lipids to counter sinking. However, there is no direct evidence for this hypothesis.[16] [17] To date, the only buoyancy mechanism successfully demonstrated in marine diatoms is the buoyancy regulation through an ionic pump.[18]
A feature of diatoms is the urea cycle, which links them evolutionarily to animals. This was discovered in research carried out by Andrew Allen, Chris Bowler and colleagues. Their findings, published in 2011, that diatoms have a functioning urea cycle was highly significant, since prior to this, the urea cycle was thought to have originated with the metazoans which appeared several hundreds of millions of years after the diatoms. Their study showed that while diatoms and animals use the urea cycle for different ends, they are seen to be evolutionally linked in such a way that animals and plants are not.[19]
Diatom cells are contained within a unique silica cell wall known as a frustule made up of two valves called thecae, that typically overlap one another.[20] The biogenic silica composing the cell wall is synthesised intracellularly by the polymerisation of silicic acid monomers. This material is then extruded to the cell exterior and added to the wall. In most species, when a diatom divides to produce two daughter cells, each cell keeps one of the two halves and grows a smaller half within it. As a result, after each division cycle, the average size of diatom cells in the population gets smaller. Once such cells reach a certain minimum size, rather than simply divide, they reverse this decline by forming an auxospore. This expands in size to give rise to a much larger cell, which then returns to size-diminishing divisions. Auxospore production is almost always linked to meiosis and sexual reproduction.
Decomposition and decay of diatoms leads to organic and inorganic (in the form of silicates) sediment, the inorganic component of which can lead to a method of analyzing past marine environments by corings of ocean floors or bay muds, since the inorganic matter is embedded in deposition of clays and silts and forms a permanent geological record of such marine strata. (See siliceous ooze).

1) Nucleus; holds the genetic material
2) Nucleolus; Location of the chromosomes
3) Golgi complex; modifies proteins and sends them out of the cell
4) Cell Wall; Outer membrane of the cell
5) Pyrenoid; center of carbon fixation
6) Chromatophore; pigment carrying membrane structure
7) Vacuoles; vesicle of a cell that contains fluid bound by a membrane
8) Cytoplasmic strands; hold the nucleus
9) Mitochondria; creates ATP (energy) for the cell
10) Valve/Striae; allows nutrients and waste in and out of the cell
The study of diatoms is a branch of phycology, and phycologists specializing in diatoms are called diatomists.
Silica uptake mechanism
The exact mechanism of transferring silica absorbed by the diatom to the cell wall is unknown, though research is still being undertaken. Much of the sequencing of diatom genes comes from the search for the mechanism of silica uptake and deposition in nano-scale patterns in the frustule. The most success in this area has come from two species, Thalassiosira pseudonana, which has become the model species, as the whole genome was sequenced and methods for genetic control were established, and Cylindrotheca fusiformis, in which the important silica deposition proteins silaffins were first discovered.[21] Silaffins, sets of polycationic peptides, were found in C. fusiformis cell walls and can generate intricate silica structures. These structures demonstrated pores of sizes characteristic to diatom patterns1. When T. pseudonana underwent genome analysis it was found that it encoded a urea cycle, including a higher number of polyamines than most genomes, as well as three distinct silica transport genes.[22] In a phylogenetic study on silica transport genes from 8 diverse groups of diatoms, silica transport was found to generally group with species.[21] This study also found structural differences between the silica transporters of pennate (bilateral symmetry) and centric (radial symmetry) diatoms. The sequences compared in this study were used to create a diverse background in order to identify residues that differentiate function in the silica deposition process. Additionally, the same study found that a number of the regions were conserved within species, likely the base structure of silica transport. These silica transport proteins are unique to diatoms, with no homologs found in other species, such as sponges or rice. The divergence of these silica transport genes is also indicative of the structure of the protein evolving from two repeated units composed of five membrane bound segments, which indicates either gene duplication or dimerization.[21] The silica deposition that takes place from the membrane bound vesicle in diatoms has been hypothesized to be a result of the activity of silaffins and long chain polyamines. This Silica Deposition Vesicle (SDV) has been characterized as an acidic compartment fused with Golgi-derived vesicles.[23] These two protein structures have been shown to create sheets of patterned silica in-vivo with irregular pores on the scale of diatom frustules. One hypothesis as to how these proteins work to create complex structure is that residues are conserved within the SDV’s, which is unfortunately difficult to identify or observe due to the limited number of diverse sequences available. Though the exact mechanism of the highly uniform deposition of silica is as yet unknown, the Thalassiosira pseudonana genes linked to silaffins are being looked to as targets for genetic control of nanoscale silica deposition.
Classification
The classification of heterokonts is still unsettled, and they may be treated as a division (or phylum), kingdom, or something in-between. Accordingly, groups like the diatoms may be ranked anywhere from class (usually called Diatomophyceae or Bacillariophyceae) to division (usually called Bacillariophyta), with corresponding changes in the ranks of their subgroups.
Diatoms are traditionally divided into two main groups that are primarily distinguished by frustule structures: the centrics and the pennates. Centric diatoms are radially symmetrically and are composed of upper and lower valves (epitheca and hypotheca); each consisting of a valve and a girdle band that can easily slide underneath each other and expand to increase cell content over the diatoms progression. The Centric diatom's cytoplasm is located along the inner surface of the shell and provides a hollow lining around the large vacuole located in the center of the cell. This large, central vacuole is filled by a fluid known as "Cell sap" which is similar to seawater but varies with specific ion content. The cytoplasmic layer is home to several organelles, like the chloroplasts and mitochondria. Before the Centric begins to expand, its nucleus can be found at the center of one of the valves and will begin to move towards the center of the cytoplasmic layer before division is complete. This type of diatom can be found with a variety of shapes and sizes which heavily depends on how which axis the shell extends from and if spines are added to the Centrics. Unlike Centric diatoms, Pennate diatoms are bilaterally not radially symmetric. Each one of their valves have openings that are slits along the raphes and their shells are typically elongated parallel to these raphes. They generate cell movement through cytoplasm that streams along the raphes, always moving along solid surfaces.
Due to the difference noted in pennate diatoms, of the presence or absence of a raphe (a longitudinal groove in the valve),[24] a more recent classification by Round, Crawford & Mann (1990)[9] divides the diatoms (as Bacillarophyta) into three classes and a number of orders:[25]
- Class Coscinodiscophyceae Round & R.M.Crawford: centric diatoms
- Anaulales Round & R.M.Crawford
- Arachnoidiscales Round
- Asterolamprales Round
- Aulacoseirales R.M.Crawford
- Biddulphiales
- Chaetocerotales Round & R.M.Crawfor
- Chrysanthemodiscales Round
- Corethrales Round & R.M.Crawford
- Coscinodiscales Round
- Cymatosirales Round & R.M.Crawford
- Ethmodiscales Round
- Hemiaulales Round & R.M.Crawford
- Leptocylindrales Round & R.M.Crawford
- Lithodesmiales
- Melosirales R.M.Crawford
- Orthoseirales R.M.Crawford
- Paraliales R.M.Crawford
- Rhizosoleniales
- Stictocyclales Round
- Stictodiscales Round & R.M.Crawford
- Thalassiosirales
- Triceratiales Round & R.M.Crawford
- Class Fragilariophyceae F.E.Round: pennate diatoms without a raphe (araphids)
- Ardissoneales F.E.Round
- Climacospheniales Round
- Cyclophorales Round & R.M.Crawford
- Fragilariales P.C.Silva
- Licmophorales Round
- Protoraphidales Round
- Rhabdonematales Round & R.M.Crawford
- Rhaphoneidales Round
- Striatellales F.E.Round
- Tabellariales Round
- Thalassionematales Round
- Toxariales Round
- Class Bacillariophyceae Haeckel, 1878, emend. D.G.Mann: pennate diatoms with a raphe (raphids)
- Achnanthales P.C.Silva
- Bacillariales Hendey
- Cymbellales D.G.Mann
- Dictyoneidales D.G.Mann
- Eunotiales P.C.Silva
- Lyrellales D.G.Mann
- Mastogloiales D.G.Mann
- Naviculales Bessey
- Rhopalodiales D.G.Mann
- Surirellales D.G.Mann
- Thalassiophysales D.G.Mann
It is probable there will be further revisions as understanding of their relationships increases. Medlin & Kaczmarska (2004) propose the following classification for the diatoms:[26]
- Bacillaryophyta
- Coscinodiscophytina
- Coscinodiscophyceae ('radial centrics')
- Bacillariophytina
- Mediophyceae ('polar centrics')
- Bacillariophyceae (pennate diatoms)
- Coscinodiscophytina

Diatoms generally range in size from 2 to 200μm,[8] and build intricate hard but porous cell walls (called frustules or tests) composed primarily of silica.[15]:25–30 This siliceous wall[27] can be highly patterned with a variety of pores, ribs, minute spines, marginal ridges and elevations; all of which can be used to delineate genera and species. The cell itself consists of two halves, each containing an essentially flat plate, or valve and marginal connecting, or girdle band. One half, the hypotheca, is slightly smaller than the other half, the epitheca. Diatom morphology varies. Although the shape of the cell is typically circular, some cells may be triangular, square, or elliptical. Their distinguishing feature is a hard mineral shell or frustule composed of opal (hydrated, polymerized silicic acid).
Cells are solitary or united into colonies of various kinds, which may be linked by siliceous structures; mucilage pads, stalks or tubes; amorphous masses of mucilage, or by threads of chitin, (polysaccharide) which are secreted through strutted processes of the cell. Major pigments of diatoms are chlorophylls a and c, beta-carotene, fucoxanthin, diatoxanthin and diadinoxanthin.[8] Diatoms are mainly photosynthetic. A few, however, are obligate heterotrophs, while others can live heterotrophically in the absence of light, provided an appropriate organic carbon source is available. Storage products are chrysolaminarin and lipids.[15]
Hoek et al. (1995)[28] also provide a comprehensive coverage of diatom taxonomy.
Ecology

Planktonic diatoms in freshwater and marine environments typically exhibit a "boom and bust" (or "bloom and bust") lifestyle. When conditions in the upper mixed layer (nutrients and light) are favourable (as at the spring), their competitive edge and rapid growth rate[12] enables them to dominate phytoplankton communities ("boom" or "bloom"). As such they are often classed as opportunistic r-strategists (i.e. those organisms whose ecology is defined by a high growth rate, r).
The freshwater diatom Didymosphenia geminata, commonly known as Didymo causes severe environmental degradation in water-courses where it blooms, producing large quantities of a brown jelly-like material called "brown snot" or "rock snot". This diatom is native to Europe and is an invasive species both in the antipodes and in parts of North America.[30][31] The problem is most frequently recorded from Australia and New Zealand.[32]
When conditions turn unfavourable, usually upon depletion of nutrients, diatom cells typically increase in sinking rate and exit the upper mixed layer ("bust"). This sinking is induced by either a loss of buoyancy control, the synthesis of mucilage that sticks diatoms cells together, or the production of heavy resting spores. Sinking out of the upper mixed layer removes diatoms from conditions unfavourable to growth, including grazer populations and higher temperatures (which would otherwise increase cell metabolism). Cells reaching deeper water or the shallow seafloor can then rest until conditions become more favourable again. In the open ocean, many sinking cells are lost to the deep, but refuge populations can persist near the thermocline.
Ultimately, diatom cells in these resting populations re-enter the upper mixed layer when vertical mixing entrains them. In most circumstances, this mixing also replenishes nutrients in the upper mixed layer, setting the scene for the next round of diatom blooms. In the open ocean (away from areas of continuous upwelling[33]), this cycle of bloom, bust, then return to pre-bloom conditions typically occurs over an annual cycle, with diatoms only being prevalent during the spring and early summer. In some locations, however, an autumn bloom may occur, caused by the breakdown of summer stratification and the entrainment of nutrients while light levels are still sufficient for growth. Since vertical mixing is increasing, and light levels are falling as winter approaches, these blooms are smaller and shorter-lived than their spring equivalents.
In the open ocean, the diatom (spring) bloom is typically ended by a shortage of silicon. Unlike other minerals, the requirement for silicon is unique to diatoms and it is not regenerated in the plankton ecosystem as efficiently as, for instance, nitrogen or phosphorus nutrients. This can be seen in maps of surface nutrient concentrations – as nutrients decline along gradients, silicon is usually the first to be exhausted (followed normally by nitrogen then phosphorus).
Because of this bloom-and-bust cycle, diatoms are believed to play a disproportionately important role in the export of carbon from oceanic surface waters[33][34] (see also the biological pump). Significantly, they also play a key role in the regulation of the biogeochemical cycle of silicon in the modern ocean.[13][29]
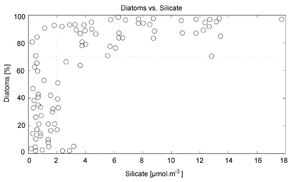
The use of silicon by diatoms is believed by many researchers to be the key to their ecological success. In a now classic study, Egge and Aksnes (1992)[35] found that diatom dominance of mesocosm communities was directly related to the availability of silicic acid — when concentrations were greater than 2 µmol m−3, they found that diatoms typically represented more than 70% of the phytoplankton community. Raven (1983)[36] noted that, relative to organic cell walls, silica frustules require less energy to synthesize (approximately 8% of a comparable organic wall), potentially a significant saving on the overall cell energy budget. Other researchers[37] have suggested that the biogenic silica in diatom cell walls acts as an effective pH buffering agent, facilitating the conversion of bicarbonate to dissolved CO2 (which is more readily assimilated). Notwithstanding the possible advantages conferred by silicon, diatoms typically have higher growth rates than other algae of a corresponding size.[12]
Diatoms occur in virtually every environment that contains water. Diatoms inhabit not only oceans, seas, lakes, and streams, but also soil and wetlands.
Life cycle

Reproduction among these organisms is primarily asexual by binary fission, with each daughter cell receiving one of the parent cell's two frustules (or theca). This is used by each daughter cell as the larger frustule (or epitheca) into which a second, small frustule (or hypotheca) is constructed.
This form of division results in a size reduction of the daughter cell that received the smaller frustule from the parent and therefore the average cell size of a diatom population decreases, until the cells are about one-third their maximum size.[8] It has been observed, however, that certain taxa have the ability to divide without causing a reduction in cell size.[38] Nonetheless, in order to restore the cell size of a diatom population for those that do endure size reduction, sexual reproduction and auxospore formation must occur.[8] Vegetative cells of diatoms are diploid (2N) and so meiosis can take place, producing male and female gametes which then fuse to form the zygote. The zygote sheds its silica theca and grows into a large sphere covered by an organic membrane, the auxospore. A new diatom cell of maximum size, the initial cell, forms within the auxospore thus beginning a new generation. Resting spores may also be formed as a response to unfavourable environmental conditions with germination occurring when conditions improve.[15]
Diatoms are mostly non-motile; however, sperm found in some species can be flagellated, though motility is usually limited to a gliding motion.[15] In centric diatoms, the small male gametes have one flagellum while the female gametes are large and non-motile (oogamous). Conversely, in pennate diatoms both gametes lack flagella (isoogamous).[8] Certain araphid species, that is pennate diatoms without a raphe, have been documented as anisogamous and are, therefore, considered to represent a transitional stage between centric and raphid pennate diatoms, diatoms with a raphe.[38]
Evolutionary history
Heterokont chloroplasts appear to derive from those of red algae, rather than directly from prokaryotes as occurred in plants. This suggests they had a more recent origin than many other algae. However, fossil evidence is scant, and only with the evolution of the diatoms themselves do the heterokonts make a serious impression on the fossil record.
The earliest known fossil diatoms date from the early Jurassic (~185 Ma ago),[39] although the molecular clock[39] and sedimentary[40] evidence suggests an earlier origin. It has been suggested that their origin may be related to the end-Permian mass extinction (~250 Ma), after which many marine niches were opened.[41] The gap between this event and the time that fossil diatoms first appear may indicate a period when diatoms were unsilicified and their evolution was cryptic.[42] Since the advent of silicification, diatoms have made a significant impression on the fossil record, with major deposits of fossil diatoms found as far back as the early Cretaceous, and some rocks (diatomaceous earth, diatomite, kieselguhr) being composed almost entirely of them.
Although the diatoms may have existed since the Triassic, the timing of their ascendancy and "take-over" of the silicon cycle occurred more recently. Prior to the Phanerozoic (before 544 Ma), it is believed that microbial or inorganic processes weakly regulated the ocean's silicon cycle.[43][44][45] Subsequently, the cycle appears dominated (and more strongly regulated) by the radiolarians and siliceous sponges, the former as zooplankton, the latter as sedentary filter-feeders primarily on the continental shelves.[46] Within the last 100 My, it is thought that the silicon cycle has come under even tighter control, and that this derives from the ecological ascendancy of the diatoms.
However, the precise timing of the "take-over" remains unclear, and different authors have conflicting interpretations of the fossil record. Some evidence, such as the displacement of siliceous sponges from the shelves,[47] suggests that this takeover began in the Cretaceous (146 Ma to 65 Ma), while evidence from radiolarians suggests "take-over" did not begin until the Cenozoic (65 Ma to present).[48] The expansion of grassland biomes and the evolutionary radiation of grasses during the Miocene is believed to have increased the flux of soluble silicon to the oceans, and it has been argued that this promoted the diatoms during the Cenozoic era.[49][50] Recent work suggests that diatom success is decoupled from the evolution of grasses, although both diatom and grassland diversity increased strongly from the middle Miocene.[51] Diatom diversity over the Cenozoic has been very sensitive to global temperature, particularly to the equator-pole temperature gradient. Warmer oceans, particularly warmer polar regions, have in the past been shown to have had substantially lower diatom diversity. Future warm oceans with enhanced polar warming, as projected in global-warming scenarios,[52] could thus in theory result in a significant loss of diatom diversity, although from current knowledge it is impossible to say if this would occur rapidly or only over many tens of thousands of years.[51]
Fossil record
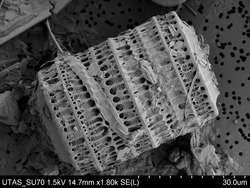
The fossil record of diatoms has largely been established through the recovery of their siliceous frustules in marine and non-marine sediments. Although diatoms have both a marine and non-marine stratigraphic record, diatom biostratigraphy, which is based on time-constrained evolutionary originations and extinctions of unique taxa, is only well developed and widely applicable in marine systems. The duration of diatom species ranges have been documented through the study of ocean cores and rock sequences exposed on land.[53] Where diatom biozones are well established and calibrated to the geomagnetic polarity time scale (e.g., Southern Ocean, North Pacific, eastern equatorial Pacific), diatom-based age estimates may be resolved to within <100,000 years, although typical age resolution for Cenozoic diatom assemblages is several hundred thousand years.
The Cretaceous record of diatoms is limited, but recent studies reveal a progressive diversification of diatom types. The Cretaceous–Paleogene extinction event, which in the oceans dramatically affected organisms with calcareous skeletons, appears to have had relatively little impact on diatom evolution.[54]

Although no mass extinctions of marine diatoms have been observed during the Cenozoic, times of relatively rapid evolutionary turnover in marine diatom assemblages occurred near the Paleocene–Eocene boundary[55] and at the Eocene–Oligocene boundary.[56] Further turnover of assemblages took place at various times between the middle Miocene and late Pliocene,[57] in response to progressive cooling of polar regions and the development of more endemic diatom assemblages. A global trend toward more delicate diatom frustules has been noted from the Oligocene to the Quaternary.[53] This coincides with an increasingly more vigorous circulation of the ocean’s surface and deep waters brought about by increasing latitudinal thermal gradients at the onset of major ice sheet expansion on Antarctica and progressive cooling through the Neogene and Quaternary towards a bipolar glaciated world. This drove the diatoms into uptaking silica more competitively (i.e., to use less silica in formation of their frustules). Increased mixing of the oceans renews silica and other nutrients necessary for diatom growth in surface waters, especially in regions of coastal and oceanic upwelling.
Diatoms preserved in lake sediments are widely used for paleoenvironmental reconstructions of Quaternary climate, especially for closed-basin lakes which experience fluctuations in water depth and salinity.
Collection
Living diatoms are often found clinging in great numbers to filamentous algae, or forming gelatinous masses on various submerged plants. Cladophora is frequently covered with Cocconeis, an elliptically shaped diatom; Vaucheria is often covered with small forms. Diatoms are frequently present as a brown, slippery coating on submerged stones and sticks, and may be seen to "stream" with river current.
The surface mud of a pond, ditch, or lagoon will almost always yield some diatoms. They can be made to emerge by filling a jar with water and mud, wrapping it in black paper and letting direct sunlight fall on the surface of the water. Within a day, the diatoms will come to the top in a scum and can be isolated.
Since diatoms form an important part of the food of molluscs, tunicates, and fishes, the alimentary tracts of these animals often yield forms that are not easily secured in other ways. Marine diatoms can be collected by direct water sampling, though benthic forms can be secured by scraping barnacles, oyster shells, and other shells.
This section uses text from Methods in Plant Histology.[58]
Expressed sequence tagging
The first insights into the properties of the Phaeodactylum tricornutum gene repertoire were described using 1,000 expressed sequence tags (ESTs).[59] Subsequently, the number of ESTs was extended to 12,000 and the diatom EST database was constructed for functional analyses.[60] These sequences have been used to make a comparative analysis between P. tricornutum and the putative complete proteomes from the green alga Chlamydomonas reinhardtii, the red alga Cyanidioschyzon merolae, and the diatom Thalassiosira pseudonana.[61] The diatom EST database now consists of over 200,000 ESTs from P. tricornutum (16 libraries) and T. pseudonana (7 libraries) cells grown in a range of different conditions, many of which correspond to different abiotic stresses.[62]
Genome sequencing
The entire genomes of the centric diatom, Thalassiosira pseudonana (32.4 Mb),[63] and the pennate diatom, Phaeodactylum tricornutum (27.4 Mb),[64] have been sequenced. Comparisons of the two fully sequenced diatom genomes finds that the P. tricornutum genome includes fewer genes (10,402 opposed to 11,776) than T. pseudonana and no major synteny (gene order) could be detected between the two genomes. T. pseudonana genes show an average of ~1.52 introns per gene as opposed to 0.79 in P. tricornutum, suggesting recent widespread intron gain in the centric diatom.[64][65] Despite relatively recent evolutionary divergence (90 million years), the extent of molecular divergence between centrics and pennates indicates rapid evolutionary rates within the Bacillariophyceae compared to other eukaryotic groups.[64] Comparative genomics also established that a specific class of transposable elements, the Diatom Copia-like retrotransposons (or CoDis), has been significantly amplified in the P. tricornutum genome with respect to T. pseudonana, constituting 5.8 and 1% of the respective genomes.[66]
Importantly, diatom genomics brought much information about the extent and dynamics of the endosymbiotic gene transfer (EGT) process. Comparison of the T. pseudonana proteins with homologs in other organisms suggested that hundreds have their closest homologs in the Plantae lineage. EGT towards diatom genomes can be illustrated by the fact that the T. pseudonana genome encodes six proteins which are most closely related to genes encoded by the Guillardia theta (cryptomonad) nucleomorph genome. Four of these genes are also found in red algal plastid genomes, thus demonstrating successive EGT from red algal plastid to red algal nucleus (nucleomorph) to heterokont host nucleus.[63] More recent phylogenomic analyses of diatom proteomes provided evidence for a prasinophyte-like endosymbiont in the common ancestor of chromalveolates as supported by the fact the 70% of diatom genes of Plantae origin are of green lineage provenance and that such genes are also found in the genome of other stramenopiles. Therefore, it was proposed that chromalveolates are the product of serial secondary endosymbiosis first with a green algae, followed by a second one with a red algae that conserved the genomic footprints of the previous but displaced the green plastid.[67] However, phylogenomic analyses of diatom proteomes and chromalveolate evolutionary history will likely take advantage of complementary genomic data from under-sequenced lineages such as red algae.
In addition to EGT, horizontal gene transfer (HGT) can occur independently of an endosymbiotic event. The publication of the P. tricornutum genome reported that at least 587 P. tricornutum genes appear to be most closely related to bacterial genes, accounting for more than 5% of the P. tricornutum proteome. About half of these are also found in the T. pseudonana genome, attesting their ancient incorporation in the diatom lineage.[64]
Photonic properties
Diatoms are often referred as "jewels of the sea" or "living opals" thanks to their photonic crystal properties.[68] It is not clear what is the biological significant of such structural coloration but it is speculated that it varies from communication, camouflage, and thermal exchange to UV protection.[69]
Forensic research
The main goal of diatom analysis in forensics is to differentiate a death by submersion from a post-mortem immersion of a body in water. Laboratory tests may reveal the presence of diatoms in the body. Since the silica-based skeletons of diatoms do not readily decay, they can sometimes be detected even in heavily decomposed bodies. As they do not occur naturally in the body, if laboratory tests show diatoms in the corpse that are of the same species found in the water where the body was recovered, then it may be good evidence of drowning as the cause of death. The blend of diatom species found in a corpse may be the same or different from the surrounding water, indicating whether the victim drowned in the same site in which the body was found.[70]
Nanotechnology research
The deposition of silica by diatoms may also prove to be of utility to nanotechnology.[71] Diatom cells repeatedly and reliably manufacture valves of various shapes and sizes, potentially allowing diatoms to manufacture micro- or nano-scale structures which may be of use in a range of devices, including: optical systems; semiconductor nanolithography; and even vehicles for drug delivery. With an appropriate artificial selection procedure, diatoms that produce valves of particular shapes and sizes might be evolved for cultivation in chemostat cultures to mass-produce nanoscale components.[72] It has also been proposed that diatoms could be used as a component of solar cells by substituting photosensitive titanium dioxide for the silicon dioxide that diatoms normally use to create their cell walls.[73] Diatom biofuel producing solar panels have also been proposed.[74]
Microbial degradation
Certain species of bacteria in oceans and lakes can accelerate the rate of dissolution of silica in dead and living diatoms by using hydrolytic enzymes to break down the organic algal material.[75][76]
See also
References
- ↑ Dangeard, P. (1933). Traite d'Algologie. Paul Lechvalier and Fils, Paris, .
- ↑ Dumortier, B.-C. (1822). Commentationes botanicae. Observations botaniques, dédiées à la Société d'Horticulture de Tournay (disponible at Algaebase). pp. [i], [1]-116, [1, tabl., err.]. Tournay: Imprimerie de Ch. Casterman-Dieu, Rue de pont No. 10.
- ↑ Rabenhorst, L. Flora europaea algarum aquae dulcis et submarinae (1864–1868). Sectio I. Algas diatomaceas complectens, cum figuris generum omnium xylographice impressis (1864). pp. 1-359. Lipsiae [Leipzig]: Apud Eduardum Kummerum.
- ↑ Haeckel, E. (1878). Das Protistenreich.
- ↑ Engler, A. & Gilg, E. (1919). Syllabus der Pflanzenfamilien: eine Übersicht über das gesamte Pflanzensystem mit besonderer Berücksichtigung der Medizinal- und Nutzpflanzen, nebst einer Übersicht über die Florenreiche und Florengebiete der Erde zum Gebrauch bei Vorlesungen und Studien über spezielle und medizinisch-pharmazeutische Botanik, 8th ed., Gebrüder Borntraeger Verlag, Berlin, 395 p.
- ↑ diá-tom-os "cut in half" (= dichó-tom-os) — diá "through" or "apart" and the root of tém-n-ō "I cut". Alternation between e and o in verb root is ablaut.
- ↑ "More on Diatoms". University of California Museum of Paleontology.
- 1 2 3 4 5 6 Grethe R. Hasle; Erik E. Syvertsen; Karen A. Steidinger; Karl Tangen (1996-01-25). "Marine Diatoms". In Carmelo R. Tomas. Identifying Marine Diatoms and Dinoflagellates. Academic Press. pp. 5–385. ISBN 978-0-08-053441-1. Retrieved 2013-11-13.
- 1 2 Frank Eric Round; R. M. Crawford; D. G. Mann (1990). The Diatoms: Biology & Morphology of the Genera. Cambridge University Press. ISBN 978-0-521-36318-1. Retrieved 2013-11-13.
- ↑ Canter-Lund, H. and Lund, J.W.G. (1995). Freshwater Algae: Their microscopic world explained, Biopress Limited. ISBN 0-948737-25-5.
- ↑ Mann, David G. (2005). "The species concept in diatoms: Evidence for morphologically distinct, sympatric gamodemes in four epipelic species". Plant Systematics and Evolution. 164: 215–37. JSTOR 23675282. doi:10.1007/BF00940439.
- 1 2 3 Furnas, Miles J. (1990). "In situ growth rates of marine phytoplankton: Approaches to measurement, community and species growth rates". Journal of Plankton Research. 12 (6): 1117–51. doi:10.1093/plankt/12.6.1117. INIST:5474600.
- 1 2 Yool, Andrew; Tyrrell, Toby (2003). "Role of diatoms in regulating the ocean's silicon cycle". Global Biogeochemical Cycles. 17 (4): n/a. Bibcode:2003GBioC..17.1103Y. doi:10.1029/2002GB002018.
- ↑ Lipps, Jere H. (1970). "Plankton Evolution". Evolution. 24: 1–22. JSTOR 2406711. doi:10.2307/2406711.
- 1 2 3 4 5 Rita A. Horner (2002). A taxonomic guide to some common marine phytoplankton. Biopress. pp. 25–30. ISBN 978-0-948737-65-7. Retrieved 2013-11-13.
- ↑ Waite, Anya; Thompson, Peter; Harrison, Paul. "Does energy control the sinking rates of marine diatoms?". Limnology and Oceanography. 37(3): 468–477. doi:10.4319/lo.1992.37.3.0468.
- ↑ Sourina, Alain (August 1982). "Form and function in marine phytoplankton". Biological Reviews. 57 (3): 347–394. doi:10.1111/j.1469-185X.1982.tb00702.x.
- ↑ Anderson, Lars W. J.; Sweeney, Beatrice M. (1 May 1977). "Diel changes in sedimentation characteristics of Ditylum brightwelli: Changes in cellular lipid and effects of respiratory inhibitors and ion-transport modifiers1". Limnology and Oceanography. 22 (3): 539–552. ISSN 1939-5590. doi:10.4319/lo.1977.22.3.0539.
- ↑ Allen, Andrew E.; Dupont, Christopher L.; Oborník, Miroslav; Horák, Aleš; Nunes-Nesi, Adriano; McCrow, John P.; Zheng, Hong; Johnson, Daniel A.; Hu, Hanhua; Fernie, Alisdair R.; Bowler, Chris (2011). "Evolution and metabolic significance of the urea cycle in photosynthetic diatoms". Nature. 473 (7346): 203–7. Bibcode:2011Natur.473..203A. PMID 21562560. doi:10.1038/nature10074. Lay summary – Science Daily (May 12, 2011).
- ↑ "Diatoms". Retrieved 13 February 2016.
- 1 2 3 Thamatrakoln, K.; Alverson, A.J.; Hildebrand, M. (2006). "COMPARATIVE SEQUENCE ANALYSIS OF DIATOM SILICON TRANSPORTERS: TOWARD A MECHANISTIC MODEL OF SILICON TRANSPORT". Journal of Phycology. 42: 822–834. doi:10.1111/j.1529-8817.2006.00233.x.
- ↑ Kröger, Nils; Deutzmann, Rainer; Manfred, Sumper (November, 1999). "Polycationic Peptides from Diatom Biosilica That Direct Silica Nanosphere Formation.". Science. 286 (5442): 1129–1132. doi:10.1126/science.286.5442.1129. Check date values in:
|date=(help) - ↑ Kroger, Nils (2007). Handbook of Biomineralization: Biological Aspects and Structure Formation. Weinheim, Germany: Wiley-VCH Verlag GmbH. pp. chapter 3.
- ↑ O.E.D. 2nd edition 2005
- ↑ "Bacillariophyceae". algaebase. M. D. Guiry. Retrieved 2013-11-11.
- ↑ Medlin, Linda K.; Kaczmarska, Irena (2004). "Evolution of the diatoms: V. Morphological and cytological support for the major clades and a taxonomic revision". Phycologia. 43 (3): 245–70. doi:10.2216/i0031-8884-43-3-245.1.
- ↑ "Glass in Nature". The Corning Museum of Glass. Retrieved 19 February 2013.
- ↑ Christiaan Van Den Hoek; D. G. Mann; H. M. Jahns (1995). Algae: An Introduction to Phycology. Cambridge University Press. ISBN 978-0-521-31687-3. Retrieved 2013-11-13.
- 1 2 Treguer, P.; Nelson, D. M.; Van Bennekom, A. J.; Demaster, D. J.; Leynaert, A.; Queguiner, B. (1995). "The Silica Balance in the World Ocean: A Reestimate". Science. 268 (5209): 375–9. Bibcode:1995Sci...268..375T. PMID 17746543. doi:10.1126/science.268.5209.375.
- ↑ Didymo, Aliens Among Us. Virtual Exhibit of the Virtual Museum of Canada
- ↑ DEP Reports Didymo Discovered in the West Branch Farmington River. Retrieved on 2014-01-15.
- ↑ "Didymo Stakeholder Update – 31 October 2008". MAF Biosecurity New Zealand www.biosecurity.govt.nz. Retrieved 2013-12-01.
- 1 2 Dugdale, R. C.; Wilkerson, F. P. (1998). "Silicate regulation of new production in the equatorial Pacific upwelling". Nature. 391 (6664): 270–273. Bibcode:1998Natur.391..270D. doi:10.1038/34630.
- ↑ Smetacek, V. S. (1985). "Role of sinking in diatom life-history cycles: Ecological, evolutionary and geological significance". Mar Biol. 84 (3): 239–251. doi:10.1007/BF00392493.
- 1 2 Egge, J. K.; Aksnes, D. L. (1992). "Silicate as regulating nutrient in phytoplankton competition" (PDF). Mar Ecol. Prog. Ser. 83: 281–289. doi:10.3354/meps083281.
- ↑ Raven, J. A. (1983). "The transport and function of silicon in plants". Biol. Rev. 58 (2): 179–207. doi:10.1111/j.1469-185X.1983.tb00385.x.
- ↑ Milligan, A. J.; Morel, F. M. M. (2002). "A proton buffering role for silica in diatoms". Science. 297 (5588): 1848–1850. Bibcode:2002Sci...297.1848M. PMID 12228711. doi:10.1126/science.1074958.
- 1 2 G. Drebes (1 January 1977). "Chapter 9: Sexuality". In Dietrich Werner. The Biology of Diatoms. Volume 13 of Botanical Monographs. University of California Press. pp. 250–283. ISBN 978-0-520-03400-6. Retrieved 2013-11-14.
- 1 2 Kooistra, Wiebe H.C.F.; Medlin, Linda K. (1996). "Evolution of the Diatoms (Bacillariophyta)". Molecular Phylogenetics and Evolution. 6 (3): 391–407. PMID 8975694. doi:10.1006/mpev.1996.0088.
- ↑ Schieber, Jürgen; Krinsley, Dave; Riciputi, Lee (2000). "Diagenetic origin of quartz silt in mudstones and implications for silica cycling". Nature. 406 (6799): 981–5. PMID 10984049. doi:10.1038/35023143.
- ↑ Medlin, L. K.; Kooistra, W. H. C. F.; Gersonde, R.; Sims, P. A.; Wellbrock, U. (1997). "Is the origin of the diatoms related to the end-Permian mass extinction?". Nova Hedwegia. 65: 1–11. hdl:10013/epic.12689.
- ↑ Raven, J. A.; Waite, A. M. (2004). "The evolution of silicification in diatoms: Inescapable sinking and sinking as escape?". New Phytologist. 162: 45–61. JSTOR 1514475. doi:10.1111/j.1469-8137.2004.01022.x.
- ↑ R. Siever; Stephen Henry Schneider; Penelope J. Boston (January 1993). "Silica in the oceans: biological-geological interplay". Scientists on Gaia. MIT Press. pp. 287–295. ISBN 978-0-262-69160-4. Retrieved 2013-11-14.
- ↑ Kidder, David L.; Erwin, Douglas H. (2001). "Secular Distribution of Biogenic Silica through the Phanerozoic: Comparison of Silica‐Replaced Fossils and Bedded Cherts at the Series Level". The Journal of Geology. 109 (4): 509–22. Bibcode:2001JG....109..509K. doi:10.1086/320794.
- ↑ Grenne, Tor; Slack, John F. (2003). "Paleozoic and Mesozoic silica-rich seawater: Evidence from hematitic chert (jasper) deposits". Geology. 31 (4): 319–22. Bibcode:2003Geo....31..319G. doi:10.1130/0091-7613(2003)031<0319:PAMSRS>2.0.CO;2. INIST:14692468.
- ↑ Racki, G; Cordey, Fabrice (2000). "Radiolarian palaeoecology and radiolarites: Is the present the key to the past?". Earth-Science Reviews. 52: 83–120. Bibcode:2000ESRv...52...83R. doi:10.1016/S0012-8252(00)00024-6.
- ↑ Maldonado, Manuel; Carmona, M. Carmen; Uriz, María J.; Cruzado, Antonio (1999). "Decline in Mesozoic reef-building sponges explained by silicon limitation". Nature. 401 (6755): 785–8. Bibcode:1999Natur.401..785M. doi:10.1038/44560. INIST:1990263.
- ↑ Harper, Howard E.; Knoll, Andrew H. (1975). "Silica, diatoms, and Cenozoic radiolarian evolution". Geology. 3 (4): 175–7. Bibcode:1975Geo.....3..175H. doi:10.1130/0091-7613(1975)3<175:SDACRE>2.0.CO;2.
- ↑ Falkowski, P. G.; Katz, Miriam E.; Knoll, Andrew H.; Quigg, Antonietta; Raven, John A.; Schofield, Oscar; Taylor, F. J. R. (2004). "The Evolution of Modern Eukaryotic Phytoplankton". Science. 305 (5682): 354–60. Bibcode:2004Sci...305..354F. PMID 15256663. doi:10.1126/science.1095964.
- ↑ Kidder, D. L.; Gierlowski-Kordesch, E. H. (2005). "Impact of Grassland Radiation on the Nonmarine Silica Cycle and Miocene Diatomite". PALAIOS. 20 (2): 198–206. JSTOR 27670327. doi:10.2110/palo.2003.p03-108.
- 1 2 Lazarus, David; Barron, John; Renaudie, Johan; Diver, Patrick; Türke, Andreas (2014). "Cenozoic Planktonic Marine Diatom Diversity and Correlation to Climate Change". PLoS ONE. 9 (1): e84857. Bibcode:2014PLoSO...984857L. PMC 3898954
 . PMID 24465441. doi:10.1371/journal.pone.0084857.
. PMID 24465441. doi:10.1371/journal.pone.0084857. - ↑ IPCC Core Writing Team, 2007. Climate Change 2007: Synthesis Report. 104.
- 1 2 Scherer, R.P., Gladenkov, A.Yu., and Barron, J.A. (2007). Methods and applications of Cenozoic marine diatom biostratigraphy. "Paleontological Society Papers" 13, 61–83
- ↑ Harwood, D.M., Nikolaev, V.A., and Winter, D.M. (2007). Cretaceous record of diatom evolution, radiation, and expansion. "Paleontological Society Papers" 13, 33–59
- ↑ Strelnikova, N. I. (1990). Evolution of diatoms during the Cretaceous and Paleogene periods. In: Simola, H. (ed.), "Proceedings of the Tenth International Diatom Symposium", Koeltz Scientific Books, Koenigstein, Germany, pp. 195–204
- ↑ Baldauf, J.G. (1993). Middle Eocene through early Miocene diatom floral turnover. In: Prothero D., Berggren, W.H., (eds.), "Eocene-Oligocene climatic and biotic evolution", Princeton University Press, Princeton, NJ, USA, pp 310–326
- ↑ Barron, J.A. (2003). Appearance and extinction of planktonic diatoms during the past 18 m.y. in the Pacific and Southern oceans. "Diatom Research" 18, 203–224
- ↑ Chamberlain, C. J. (1901) Methods in Plant Histology, University of Chicago Press, USA
- ↑ Scala, S.; Carels, N; Falciatore, A; Chiusano, M. L.; Bowler, C (2002). "Genome Properties of the Diatom Phaeodactylum tricornutum". Plant Physiology. 129 (3): 993–1002. PMC 166495
 . PMID 12114555. doi:10.1104/pp.010713.
. PMID 12114555. doi:10.1104/pp.010713. - ↑ Maheswari, U.; Montsant, A; Goll, J; Krishnasamy, S; Rajyashri, K. R.; Patell, V. M.; Bowler, C (2004). "The Diatom EST Database". Nucleic Acids Research. 33 (Database issue): D344–7. PMC 540075
 . PMID 15608213. doi:10.1093/nar/gki121.
. PMID 15608213. doi:10.1093/nar/gki121. - ↑ Montsant, A.; Jabbari, K; Maheswari, U; Bowler, C (2005). "Comparative Genomics of the Pennate Diatom Phaeodactylum tricornutum". Plant Physiology. 137 (2): 500–13. PMC 1065351
 . PMID 15665249. doi:10.1104/pp.104.052829.
. PMID 15665249. doi:10.1104/pp.104.052829. - ↑ Maheswari, U.; Mock, T.; Armbrust, E. V.; Bowler, C. (2009). "Update of the Diatom EST Database: A new tool for digital transcriptomics". Nucleic Acids Research. 37 (Database issue): D1001–5. PMC 2686495
 . PMID 19029140. doi:10.1093/nar/gkn905.
. PMID 19029140. doi:10.1093/nar/gkn905. - 1 2 Armbrust, E. V.; Berges, John A.; Bowler, Chris; Green, Beverley R.; Martinez, Diego; Putnam, Nicholas H.; Zhou, Shiguo; Allen, Andrew E.; Apt, Kirk E.; Bechner, Michael; Brzezinski, Mark A.; Chaal, Balbir K.; Chiovitti, Anthony; Davis, Aubrey K.; Demarest, Mark S.; Detter, J. Chris; Glavina, Tijana; Goodstein, David; Hadi, Masood Z.; Hellsten, Uffe; Hildebrand, Mark; Jenkins, Bethany D.; Jurka, Jerzy; Kapitonov, Vladimir V.; Kröger, Nils; Lau, Winnie W. Y.; Lane, Todd W.; Larimer, Frank W.; Lippmeier, J. Casey; et al. (2004). "The Genome of the Diatom Thalassiosira Pseudonana: Ecology, Evolution, and Metabolism". Science. 306 (5693): 79–86. Bibcode:2004Sci...306...79A. PMID 15459382. doi:10.1126/science.1101156.
- 1 2 3 4 Bowler, Chris; Allen, Andrew E.; Badger, Jonathan H.; Grimwood, Jane; Jabbari, Kamel; Kuo, Alan; Maheswari, Uma; Martens, Cindy; Maumus, Florian; Otillar, Robert P.; Rayko, Edda; Salamov, Asaf; Vandepoele, Klaas; Beszteri, Bank; Gruber, Ansgar; Heijde, Marc; Katinka, Michael; Mock, Thomas; Valentin, Klaus; Verret, Fréderic; Berges, John A.; Brownlee, Colin; Cadoret, Jean-Paul; Chiovitti, Anthony; Choi, Chang Jae; Coesel, Sacha; De Martino, Alessandra; Detter, J. Chris; Durkin, Colleen; et al. (2008). "The Phaeodactylum genome reveals the evolutionary history of diatom genomes". Nature. 456 (7219): 239–44. Bibcode:2008Natur.456..239B. PMID 18923393. doi:10.1038/nature07410.
- ↑ Roy, S. W.; Penny, D. (2007). "A Very High Fraction of Unique Intron Positions in the Intron-Rich Diatom Thalassiosira pseudonana Indicates Widespread Intron Gain". Molecular Biology and Evolution. 24 (7): 1447–57. PMID 17350938. doi:10.1093/molbev/msm048.
- ↑ Maumus, Florian; Allen, Andrew E; Mhiri, Corinne; Hu, Hanhua; Jabbari, Kamel; Vardi, Assaf; Grandbastien, Marie-Angèle; Bowler, Chris (2009). "Potential impact of stress activated retrotransposons on genome evolution in a marine diatom". BMC Genomics. 10: 624. PMC 2806351
 . PMID 20028555. doi:10.1186/1471-2164-10-624.
. PMID 20028555. doi:10.1186/1471-2164-10-624. - ↑ Moustafa, A.; Beszteri, B.; Maier, U. G.; Bowler, C.; Valentin, K.; Bhattacharya, D. (2009). "Genomic Footprints of a Cryptic Plastid Endosymbiosis in Diatoms". Science. 324 (5935): 1724–6. Bibcode:2009Sci...324.1724M. PMID 19556510. doi:10.1126/science.1172983.
- ↑ Parker, Andrew R.; Townley, Helen E. (2007). "Biomimetics of photonic nanostructures". Nature Nanotechnology. 2 (6): 347–53. Bibcode:2007NatNa...2..347P. PMID 18654305. doi:10.1038/nnano.2007.152.
- ↑ Gordon, Richard; Losic, Dusan; Tiffany, Mary Ann; Nagy, Stephen S.; Sterrenburg, Frithjof A.S. (2009). "The Glass Menagerie: Diatoms for novel applications in nanotechnology". Trends in Biotechnology. 27 (2): 116–27. PMID 19167770. doi:10.1016/j.tibtech.2008.11.003.
- ↑ Auer, Antti (1991). "Qualitative Diatom Analysis as a Tool to Diagnose Drowning". The American Journal of Forensic Medicine and Pathology. 12 (3): 213–8. PMID 1750392. doi:10.1097/00000433-199109000-00009.
- ↑ Bradbury, J. (2004). "Nature's Nanotechnologists: Unveiling the Secrets of Diatoms". PLoS Biology. 2 (10): 1512–1515. PMC 521728
 . PMID 15486572. doi:10.1371/journal.pbio.0020306.
. PMID 15486572. doi:10.1371/journal.pbio.0020306. 
- ↑ Drum, Ryan W.; Gordon, Richard (2003). "Star Trek replicators and diatom nanotechnology". Trends in Biotechnology. 21 (8): 325–8. PMID 12902165. doi:10.1016/S0167-7799(03)00169-0.
- ↑ Johnson, R.C. (9 April 2009). "Diatoms could triple solar cell efficiency". EE Times. Retrieved 13 April 2009.
- ↑ Ramachandra, T. V.; Mahapatra, Durga Madhab; b, Karthick; Gordon, Richard (2009). "Milking Diatoms for Sustainable Energy: Biochemical Engineering versus Gasoline-Secreting Diatom Solar Panels". Industrial & Engineering Chemistry Research. 48 (19): 8769–88. doi:10.1021/ie900044j.
- ↑ Azam, Farooq; Bidle, Kay D. (1999). "Accelerated dissolution of diatom silica by marine bacterial assemblages". Nature. 397 (6719): 508–12. Bibcode:1999Natur.397..508B. doi:10.1038/17351. INIST:1755031.
- ↑ Zakharova, Yulia R.; Galachyants, Yuri P.; Kurilkina, Maria I.; Likhoshvay, Alexander V.; Petrova, Darya P.; Shishlyannikov, Sergey M.; Ravin, Nikolai V.; Mardanov, Andrey V.; Beletsky, Alexey V.; Likhoshway, Yelena V. (2013). "The Structure of Microbial Community and Degradation of Diatoms in the Deep Near-Bottom Layer of Lake Baikal". PLoS ONE. 8 (4): e59977. Bibcode:2013PLoSO...859977Z. PMC 3613400
 . PMID 23560063. doi:10.1371/journal.pone.0059977.
. PMID 23560063. doi:10.1371/journal.pone.0059977.
External links
| Wikispecies has information related to: Diatoms |
| Wikimedia Commons has media related to Diatoms. |
- Catalogue of Diatom Names, California Academy of Sciences
- Diatom Genome, Joint Genome Institute
- Diatom EST database, École Normale Supérieure
- Plankton*Net, taxonomic database including images of diatom species
- Life History and Ecology of Diatoms, University of California Museum of Paleontology
- Diatoms: 'Nature's Marbles', Eureka site, University of Bergen
- Diatom life history and ecology, Microfossil Image Recovery and Circulation for Learning and Education (MIRACLE), University College London
- Diatom page, Royal Botanic Garden Edinburgh
- Geometry and Pattern in Nature 3: The holes in radiolarian and diatom tests
- Diatom QuickFacts, Monterey Bay Aquarium Research Institute
- Algae image database Academy of Natural Sciences of Philadelphia (ANSP)
- Diatom taxa Academy of Natural Sciences of Philadelphia (ANSP)
