Dialkylbiaryl phosphine ligands
Dialkylbiaryl phosphine ligands were first described by Stephen L. Buchwald in 1998 for their use in palladium-catalyzed coupling reactions to form carbon-nitrogen and carbon-carbon bonds.[1] Until this point, previously reported ligands such as P(o-Tol)3 required harsh conditions to catalyze a limited scope of C-N bond forming reactions. Since this initial report, extensive work has been done by both Buchwald and other laboratories to develop ligands of this type which can catalyze a wide range of reactions including Buchwald-Hartwig amination, etherification, arylation, Negishi cross-coupling, and Suzuki-Miyaura cross-coupling.[2] Today, these ligands are widely used in both academia and industry.
General features
All of the Buchwald ligands are air-stable crystalline solids. Many can be bought commercially or synthesized in only a few steps from inexpensive starting materials. One pot protocols have been developed for the synthesis of these ligands and have been employed on >10 kg scales[3][4]
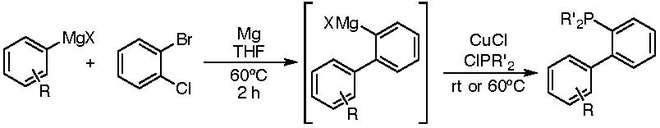
Both the steric bulk and electron-rich nature of these ligands have been attributed to their enhanced catalytic activity over other ligands in Palladium-catalyzed coupling reactions. Extensive experimentation by the Buchwald group has shown that minor changes to the structure of these ligands can dramatically alter their catalytic activity in cross coupling reactions with different substrates. This has led to the evolution of multiple ligands that are tailored for specific trasformations.[5]

DavePhos

DavePhos is the first reported dialkylbiaryl phosphine ligand and was initially used in mild Pd-catalyzed Suzuki-Miyaura cross-coupling reactions as well as Buchwald-Hartwig aminations.[6] This ligand has additionally been used to catalyze a wide array of reactions, including the -arylation of ketones[7] and esters,[8] borylation of aryl chlorides,[9] and the arylation of indoles.[10]
Many additional modified versions of DavePhos have been synthesized. tBuDavePhos has been shown to be an even more reactive variant of DavePhos in the room temperature Suzuki-Miyaura coupling of aryl bromides and chlorides.[11] The biphenyl equivalent (PhDavePhos) is also available as an alternative catalyst in Suzuki-Miyaura couplings.
SPhos

SPhos was first reported in 2004 as an extremely competent ligand in catalyzing Suzuki-Miyaura coupling reactions[12] This ligand enables the cross-coupling of heteroaryl, electron-rich and electron-poor aryl, and vinylboronic acids with a variety of aryl and heteroaryl halides under mild reaction conditions. SPhos has also been used in the Pd-catalyzed borylation of aryl and heteroaryl chlorides.[13]
3-Sulfonate variants of this ligand (sSPhos) have been shown to catalyze Suzuki-Miyaura couplings in aqueous media.[14] One example of the application of SPhos to academia was its use in the 8 step total synthesis of (±)-geigerin.[15]

XPhos

XPhos was first used in 2003 for the general amination and amidation of arenesulfonates and aryl halides.[16] XPhos has also been used in the Pd catalyzed borylation of aryl and heteroaryl chlorides [17]
Modified versions of XPhos have also been reported. The more hindered tBuXPhos and Me4tButylXPhos has been employed in the formation of diaryl ethers.[18] Incorporation of a sulfonate group at the 4-position allows this ligand to be used for Sonogashira couplings in aqueous biphasic solvents.[19]
BrettPhos
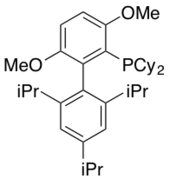
BrettPhos was originally reported in 2008 for the Pd-catalyzed amination of aryl mesylates and aryl halides.[20] This ligand helps promote the coupling of weak nucleophiles with aryl halides. Notably, this ligand is highly selective for the monoarylation of primary amines with minimal formation of the diarylated product. BrettPhos is also chemoselective for primary amines over secondary amines. Other applications of BrettPhos in catalysis include trifluoromethylation of aryl chlorides,[21] the formation of aryl trifluoromethyl sulfides,[22] and Suzuki-Miyaura Cross-couplings.[23]
Several modified versions of BrettPhos are also commercially available. tBuBrettPhos has been shown to be a robust ligand in the catalytic conversion of aryl triflates and aryl bromides to aryl fluorides[24] as well as the synthesis of aromatic nitro compounds.[25] The extremely bulky AdBrettPhos can be used in the amidation of five-membered heterocyclic halides that contain multiple heteroatoms (such as haloimidazoles and halopyrazoles).[26]
JohnPhos

Named after John P. Wolfe, JohnPhos was originally used in 1999 in Pd catalyzed Suzuki-Miyaura reactions with aryl bromides and chlorides.[27] It allows hindered substrates to be reacted at room temperature with extremely low catalyst loading. Since then, this ligand has been utilized in multiple reactions including the amination of a wide range of aryl halides and triflates[28][29] as well as the arylation of thiophenes.[30]
Several modified version of this ligand including PhJohnPhos and CyJohnPhos have also been investigated for their use in Pd-catalyzed cross-couplings.
RuPhos
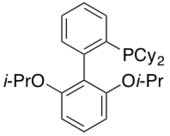
RuPhos was first reported in 2004 as part of a highly reactive catalyst system for the Pd-catalyzed Negishi coupling of organozincs with aryl halides.[31] This ligands allows both extremely hindered substrates as well as substrates with a wide range of functional groups to undergo this reaction. This ligand has also been shown to be effective at catalyzing reactions such as the trifluoromethylation of aryl chlorides[32] and aminations of aryl halides.[33]
MePhos
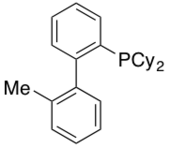
First reported in 1999, MePhos has been shown to be an equally competent ligand in the Pd-catalyzed Suzuki-Miyaura to that of DavePhos and JohnPhos.[34] It can also form the active catalyst in the formation of aryl ketones.[35] Variants of this ligand including tBuMePhos are also commercially available.
Coworkers at Amgen found that the late stage Suzuki cross coupling (in route to a novel p38 MAP Kinase Inhibitor) was best catalyzed by a Pd2(dba)3/MePhos catalytic system. This reaction was preformed on a kilogram scale and no specific palladium-removal treatment was required as the excess imidazole present in the final amide coupling step coordinated to the Pd and generated a removable byproduct.[36]

CPhos
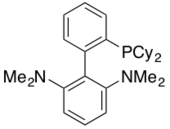
CPhos has been utilized as a highly active ligand in the Pd-catalyzed Negishi Coupling of Secondary alkylzinc reagents with aryl halides.[37]
AlPhos

AlPhos is one of the newest commercially available Dialkylbiaryl phosphine ligands. Reported in 2015, this ligand allows for the mild Pd-catalyzed fluorination of aryl- and heteroaryl triflates.[38]
See also
References
- ↑ Old, David W.; Wolfe, John P.; Buchwald, Stephen L. (September 1998). "A Highly Active Catalyst for Palladium-Catalyzed Cross-Coupling Reactions: Room-Temperature Suzuki Couplings and Amination of Unactivated Aryl Chlorides". Journal of the American Chemical Society. 120 (37): 9722–9723. doi:10.1021/ja982250+ (inactive 2017-04-22).
- ↑ Surry, David S.; Buchwald, Stephen L. (2011). "Dialkylbiaryl phosphines in Pd-catalyzed amination: a user's guide". Chem. Sci. 2 (1): 27–50. ISSN 2041-6539. PMID 22432049. doi:10.1039/C0SC00331J.
- ↑ Martin, Ruben; Buchwald, Stephen L. (18 November 2008). "Palladium-Catalyzed Suzuki−Miyaura Cross-Coupling Reactions Employing Dialkylbiaryl Phosphine Ligands". Accounts of Chemical Research. 41 (11): 1461–1473. ISSN 0001-4842. doi:10.1021/ar800036s.
- ↑ Kaye, Steven; Fox, Joseph M.; Hicks, Frederick A.; Buchwald, Stephen L. (31 December 2001). "The Use of Catalytic Amounts of CuCl and Other Improvements in the Benzyne Route to Biphenyl-Based Phosphine Ligands". Advanced Synthesis & Catalysis. 343 (8): 789–794. ISSN 1615-4169. doi:10.1002/1615-4169(20011231)343:83.0.CO;2-A (inactive 2017-04-22).
- ↑ Martin, Ruben; Buchwald, Stephen L. (18 November 2008). "Palladium-Catalyzed Suzuki−Miyaura Cross-Coupling Reactions Employing Dialkylbiaryl Phosphine Ligands". Accounts of Chemical Research. 41 (11): 1461–1473. ISSN 0001-4842. doi:10.1021/ar800036s.
- ↑ Old, David W.; Wolfe, John P.; Buchwald, Stephen L. (September 1998). "A Highly Active Catalyst for Palladium-Catalyzed Cross-Coupling Reactions: Room-Temperature Suzuki Couplings and Amination of Unactivated Aryl Chlorides". Journal of the American Chemical Society. 120 (37): 9722–9723. doi:10.1021/ja982250+ (inactive 2017-04-22).
- ↑ Fox, Joseph M.; Huang, Xiaohua; Chieffi, André; Buchwald, Stephen L. (1 February 2000). "Highly Active and Selective Catalysts for the Formation of α-Aryl Ketones". Journal of the American Chemical Society. 122 (7): 1360–1370. ISSN 0002-7863. doi:10.1021/ja993912d.
- ↑ . doi:10.1021/ja010797+ (inactive 2017-04-22) http://pubs.acs.org/doi/abs/10.1021/ja010797%2B?journalCode=jacsat&quickLinkVolume=123&quickLinkPage=7996&selectedTab=citation&volume=123. Missing or empty
|title=(help) - ↑ Billingsley, Kelvin L.; Barder, Timothy E.; Buchwald, Stephen L. (9 July 2007). "Palladium-Catalyzed Borylation of Aryl Chlorides: Scope, Applications, and Computational Studies". Angewandte Chemie International Edition. 46 (28): 5359–5363. ISSN 1521-3773. doi:10.1002/anie.200701551.
- ↑ Old, David W.; Harris, Michele C.; Buchwald, Stephen L. (1 May 2000). "Efficient Palladium-Catalyzed N-Arylation of Indoles". Organic Letters. 2 (10): 1403–1406. ISSN 1523-7060. doi:10.1021/ol005728z.
- ↑ Wolfe, John P.; Singer, Robert A.; Yang, Bryant H.; Buchwald, Stephen L. (1 October 1999). "Highly Active Palladium Catalysts for Suzuki Coupling Reactions". Journal of the American Chemical Society. 121 (41): 9550–9561. ISSN 0002-7863. doi:10.1021/ja992130h.
- ↑ Walker, Shawn D.; Barder, Timothy E.; Martinelli, Joseph R.; Buchwald, Stephen L. (26 March 2004). "A Rationally Designed Universal Catalyst for Suzuki–Miyaura Coupling Processes". Angewandte Chemie International Edition. 43 (14): 1871–1876. ISSN 1521-3773. doi:10.1002/anie.200353615.
- ↑ Billingsley, Kelvin L.; Barder, Timothy E.; Buchwald, Stephen L. (9 July 2007). "Palladium-Catalyzed Borylation of Aryl Chlorides: Scope, Applications, and Computational Studies". Angewandte Chemie International Edition. 46 (28): 5359–5363. ISSN 1521-3773. doi:10.1002/anie.200701551.
- ↑ Anderson, Kevin W.; Buchwald, Stephen L. (26 September 2005). "General Catalysts for the Suzuki–Miyaura and Sonogashira Coupling Reactions of Aryl Chlorides and for the Coupling of Challenging Substrate Combinations in Water". Angewandte Chemie International Edition. 44 (38): 6173–6177. ISSN 1521-3773. doi:10.1002/anie.200502017.
- ↑ Carret, Sébastien; Deprés, Jean-Pierre (10 September 2007). "Access to Guaianolides: Highly Efficient Stereocontrolled Total Synthesis of (±)-Geigerin". Angewandte Chemie International Edition. 46 (36): 6870–6873. ISSN 1521-3773. doi:10.1002/anie.200702031.
- ↑ Huang, Xiaohua; Anderson, Kevin W.; Zim, Danilo; Jiang, Lei; Klapars, Artis; Buchwald, Stephen L. (1 June 2003). "Expanding Pd-Catalyzed C−N Bond-Forming Processes: The First Amidation of Aryl Sulfonates, Aqueous Amination, and Complementarity with Cu-Catalyzed Reactions". Journal of the American Chemical Society. 125 (22): 6653–6655. ISSN 0002-7863. doi:10.1021/ja035483w.
- ↑ Billingsley, Kelvin L.; Barder, Timothy E.; Buchwald, Stephen L. (9 July 2007). "Palladium-Catalyzed Borylation of Aryl Chlorides: Scope, Applications, and Computational Studies". Angewandte Chemie International Edition. 46 (28): 5359–5363. ISSN 1521-3773. doi:10.1002/anie.200701551.
- ↑ Burgos, Carlos H.; Barder, Timothy E.; Huang, Xiaohua; Buchwald, Stephen L. (26 June 2006). "Significantly Improved Method for the Pd-Catalyzed Coupling of Phenols with Aryl Halides: Understanding Ligand Effects". Angewandte Chemie International Edition. 45 (26): 4321–4326. ISSN 1521-3773. doi:10.1002/anie.200601253.
- ↑ Anderson, Kevin W.; Buchwald, Stephen L. (26 September 2005). "General Catalysts for the Suzuki–Miyaura and Sonogashira Coupling Reactions of Aryl Chlorides and for the Coupling of Challenging Substrate Combinations in Water". Angewandte Chemie International Edition. 44 (38): 6173–6177. ISSN 1521-3773. doi:10.1002/anie.200502017.
- ↑ Fors, Brett P.; Watson, Donald A.; Biscoe, Mark R.; Buchwald, Stephen L. (15 October 2008). "A Highly Active Catalyst for Pd-Catalyzed Amination Reactions: Cross-Coupling Reactions Using Aryl Mesylates and the Highly Selective Monoarylation of Primary Amines Using Aryl Chlorides". Journal of the American Chemical Society. 130 (41): 13552–13554. ISSN 0002-7863. doi:10.1021/ja8055358.
- ↑ Cho, Eun Jin; Senecal, Todd D.; Kinzel, Tom; Zhang, Yong; Watson, Donald A.; Buchwald, Stephen L. (25 June 2010). "The Palladium-Catalyzed Trifluoromethylation of Aryl Chlorides". Science. 328 (5986): 1679–1681. Bibcode:2010Sci...328.1679C. ISSN 0036-8075. doi:10.1126/science.1190524.
- ↑ Teverovskiy, Georgiy; Surry, David S.; Buchwald, Stephen L. (1 August 2011). "Pd-Catalyzed Synthesis of Ar-SCF3 Compounds under Mild Conditions". Angewandte Chemie International Edition. 50 (32): 7312–7314. ISSN 1521-3773. doi:10.1002/anie.201102543.
- ↑ Bhayana, Brijesh; Fors, Brett P.; Buchwald, Stephen L. (3 September 2009). "A Versatile Catalyst System for Suzuki−Miyaura Cross-Coupling Reactions of C(sp2)-Tosylates and Mesylates". Organic Letters. 11 (17): 3954–3957. ISSN 1523-7060. doi:10.1021/ol9015892.
- ↑ Watson, Donald A.; Su, Mingjuan; Teverovskiy, Georgiy; Zhang, Yong; García-Fortanet, Jorge; Kinzel, Tom; Buchwald, Stephen L. (25 September 2009). "Formation of ArF from LPdAr(F): Catalytic Conversion of Aryl Triflates to Aryl Fluorides". Science. 325 (5948): 1661–1664. Bibcode:2009Sci...325.1661W. ISSN 0036-8075. doi:10.1126/science.1178239.
- ↑ Fors, Brett P.; Buchwald, Stephen L. (16 September 2009). "Pd-Catalyzed Conversion of Aryl Chlorides, Triflates, and Nonaflates to Nitroaromatics". Journal of the American Chemical Society. 131 (36): 12898–12899. ISSN 0002-7863. doi:10.1021/ja905768k.
- ↑ Su, Mingjuan; Buchwald, Stephen L. (7 May 2012). "A Bulky Biaryl Phosphine Ligand Allows for Palladium-Catalyzed Amidation of Five-Membered Heterocycles as Electrophiles". Angewandte Chemie International Edition. 51 (19): 4710–4713. ISSN 1521-3773. doi:10.1002/anie.201201244.
- ↑ Wolfe, John P.; Singer, Robert A.; Yang, Bryant H.; Buchwald, Stephen L. (1 October 1999). "Highly Active Palladium Catalysts for Suzuki Coupling Reactions". Journal of the American Chemical Society. 121 (41): 9550–9561. ISSN 0002-7863. doi:10.1021/ja992130h.
- ↑ Wolfe, John P.; Tomori, Hiroshi; Sadighi, Joseph P.; Yin, Jingjun; Buchwald, Stephen L. (1 February 2000). "Simple, Efficient Catalyst System for the Palladium-Catalyzed Amination of Aryl Chlorides, Bromides, and Triflates". The Journal of Organic Chemistry. 65 (4): 1158–1174. ISSN 0022-3263. doi:10.1021/jo991699y.
- ↑ Surry, David S.; Buchwald, Stephen L. (11 August 2008). "Biaryl Phosphane Ligands in Palladium-Catalyzed Amination". Angewandte Chemie International Edition. 47 (34): 6338–6361. ISSN 1521-3773. doi:10.1002/anie.200800497.
- ↑ Okazawa, Toru; Satoh, Tetsuya; Miura, Masahiro; Nomura, Masakatsu (1 May 2002). "Palladium-Catalyzed Multiple Arylation of Thiophenes". Journal of the American Chemical Society. 124 (19): 5286–5287. ISSN 0002-7863. doi:10.1021/ja0259279.
- ↑ Milne, Jacqueline E.; Buchwald, Stephen L. (1 October 2004). "An Extremely Active Catalyst for the Negishi Cross-Coupling Reaction". Journal of the American Chemical Society. 126 (40): 13028–13032. ISSN 0002-7863. doi:10.1021/ja0474493.
- ↑ Cho, Eun Jin; Senecal, Todd D.; Kinzel, Tom; Zhang, Yong; Watson, Donald A.; Buchwald, Stephen L. (25 June 2010). "The Palladium-Catalyzed Trifluoromethylation of Aryl Chlorides". Science. 328 (5986): 1679–1681. Bibcode:2010Sci...328.1679C. ISSN 0036-8075. doi:10.1126/science.1190524.
- ↑ Charles, Mark D.; Schultz, Phillip; Buchwald, Stephen L. (1 September 2005). "Efficient Pd-Catalyzed Amination of Heteroaryl Halides". Organic Letters. 7 (18): 3965–3968. ISSN 1523-7060. doi:10.1021/ol0514754.
- ↑ Wolfe, John P.; Singer, Robert A.; Yang, Bryant H.; Buchwald, Stephen L. (1 October 1999). "Highly Active Palladium Catalysts for Suzuki Coupling Reactions". Journal of the American Chemical Society. 121 (41): 9550–9561. ISSN 0002-7863. doi:10.1021/ja992130h.
- ↑ Fox, Joseph M.; Huang, Xiaohua; Chieffi, André; Buchwald, Stephen L. (1 February 2000). "Highly Active and Selective Catalysts for the Formation of α-Aryl Ketones". Journal of the American Chemical Society. 122 (7): 1360–1370. ISSN 0002-7863. doi:10.1021/ja993912d.
- ↑ Thiel, Oliver; Achmatowicz, Michal; Milburn, Robert (11 June 2012). "Process Research and Development for Heterocyclic p38 MAP Kinase Inhibitors". Synlett. 23 (11): 1564–1574. doi:10.1055/s-0031-1290425.
- ↑ Han, Chong; Buchwald, Stephen L. (10 June 2009). "Negishi Coupling of Secondary Alkylzinc Halides with Aryl Bromides and Chlorides". Journal of the American Chemical Society. 131 (22): 7532–7533. ISSN 0002-7863. doi:10.1021/ja902046m.
- ↑ Sather, Aaron C.; Lee, Hong Geun; De La Rosa, Valentina Y.; Yang, Yang; Müller, Peter; Buchwald, Stephen L. (21 October 2015). "A Fluorinated Ligand Enables Room-Temperature and Regioselective Pd-Catalyzed Fluorination of Aryl Triflates and Bromides". Journal of the American Chemical Society. 137 (41): 13433–13438. ISSN 0002-7863. doi:10.1021/jacs.5b09308.