Detection limit
In analytical chemistry, the detection limit, lower limit of detection, or LOD (limit of detection), is the lowest quantity of a substance that can be distinguished from the absence of that substance (a blank value) within a stated confidence limit (generally 1%).[1][2] The detection limit is estimated from the mean of the blank, the standard deviation of the blank and some confidence factor. Another consideration that affects the detection limit is the accuracy of the model used to predict concentration from the raw analytical signal.
There are a number of different "detection limits" that are commonly used. These include the instrument detection limit (IDL), the method detection limit (MDL), the practical quantification limit (PQL), and the limit of quantification (LOQ). Even when the same terminology is used, there can be differences in the LOD according to nuances of what definition is used and what type of noise contributes to the measurement and calibration.[3]
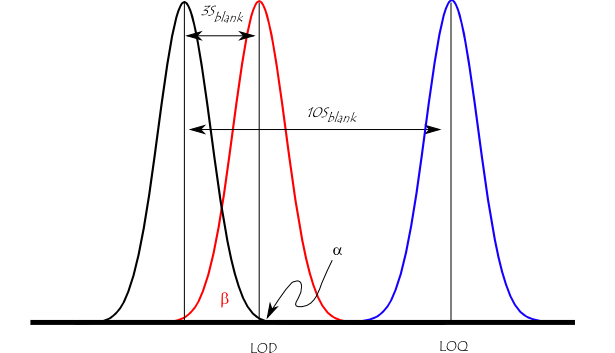
The figure below illustrates the relationship between the blank, the limit of detection (LOD), and the limit of quantification (LOQ) by showing the probability density function for normally distributed measurements at the blank, at the LOD defined as 3 * standard deviation of the blank, and at the LOQ defined as 10 * standard deviation of the blank. For a signal at the LOD, the alpha error (probability of false positive) is small (1%). However, the beta error (probability of a false negative) is 50% for a sample that has a concentration at the LOD (red line). This means a sample could contain an impurity at the LOD, but there is a 50% chance that a measurement would give a result less than the LOD. At the LOQ (blue line), there is minimal chance of a false negative.
Instrument detection limit
Most analytical instruments produce a signal even when a blank (matrix without analyte) is analyzed. This signal is referred to as the noise level. The IDL is the analyte concentration that is required to produce a signal greater than three times the standard deviation of the noise level. This may be practically measured by analyzing 8 or more standards at the estimated IDL then calculating the standard deviation from the measured concentrations of those standards. The detection limit (according to IUPAC) is the smallest concentration or absolute amount of analyte that has a signal significantly larger than the signal arising from a reagent blank. Mathematically, the analyte’s signal at the detection limit (Sdl) is given by: Sdl = Sreag + 3 * σreag
where Sreag is the signal for a reagent blank, σreag is the known standard deviation for the reagent blank’s signal.
Other approaches for defining the detection limit have also been developed. In atomic absorption spectrometry usually the detection limit is determined for a certain element by analyzing a diluted solution of this element and recording the corresponding absorbances. The experiment is repeated for 10 times. The 3σ of the recorded absorbance signal can be considered as the detection limit for the specific element under the experimental conditions used – wavelength, type of flame, instrument.
Method detection limit
Oftentimes there is more to the analytical method than just performing a reaction or submitting it to direct analysis. For example, it might be necessary to heat a sample that is to be analyzed for a particular metal with the addition of acid first (this is called digestion). The sample may also be diluted or concentrated prior to analysis on an instrument. Additional steps in an analysis add additional opportunities for error. Since detection limits are defined in terms of error, this will naturally increase the measured detection limit. This detection limit (with all steps of the analysis included) is called the MDL. The practical method for determining the MDL is to analyze 7 samples of concentration near the expected limit of detection. The standard deviation is then determined. The one-sided t-distribution is determined and multiplied versus the determined standard deviation. For seven samples (with six degrees of freedom) the t value for a 99% confidence interval is 3.14. Rather than performing the complete analysis of seven identical samples, if the Instrument Detection Limit is known, the MDL may be estimated by multiplying the Instrument Detection Limit or Lower Level of Detection by the dilution prior to analyzing the sample solution on the instrument. This estimation, however, ignores any uncertainty that arises from performing the sample preparation and will therefore probably underestimate the true MDL.
Limit of reporting
The LOQ is the limit at which the difference between two distinct values can be reasonably discerned. The LOQ may be drastically different between laboratories so another detection limit is commonly used that is referred to as the Practical Quantification Limit (PQL).
References
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "detection limit".
- ↑ MacDougall, Daniel; Crummett, Warren B.; et al. (1980), "Guidelines for Data Acquisition and Data Quality Evaluation in Environmental Chemistry", Analytical Chemistry, 52: 2242–49, doi:10.1021/ac50064a004
- ↑ Long, Gary L.; Winefordner, J. D. (1983), "Linearization of electron capture detector response to strongly responding compounds", Anal. Chem., 55 (7): 713A–724A, doi:10.1021/ac00255a030
External links
- Interactive Java applet to illustrate some basic ideas of the limit of detection problem
- Downloads of articles (a.o. harmonization of concepts by ISO and IUPAC) and an extensive list of references