Denitrification
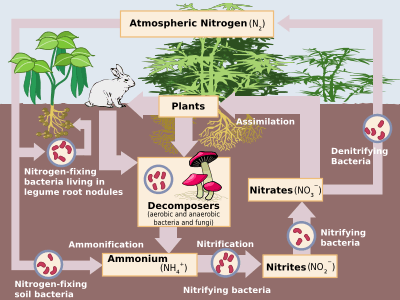
Denitrification is a microbially facilitated process where nitrate is reduced and ultimately produces molecular nitrogen (N2) through a series of intermediate gaseous nitrogen oxide products. Facultative anaerobic bacteria perform denitrification as a type of respiration that reduces oxidized forms of nitrogen in response to the oxidation of an electron donor such as organic matter. The preferred nitrogen electron acceptors in order of most to least thermodynamically favorable include nitrate (NO3−), nitrite (NO2−), nitric oxide (NO), nitrous oxide (N2O) finally resulting in the production of dinitrogen (N2) completing the nitrogen cycle. Denitrifying microbes require a very low oxygen concentration of less than 10%, as well as organic C for energy. Since denitrification can remove NO3−, reducing its leaching to groundwater, it can be strategically used to treat sewage or animal residues of high nitrogen content. Denitrification can leak N2O, which is an ozone-depleting substance and a greenhouse gas that can have a considerable influence on global warming.
The process is performed primarily by heterotrophic bacteria (such as Paracoccus denitrificans and various pseudomonads),[1] although autotrophic denitrifiers have also been identified (e.g., Thiobacillus denitrificans).[2] Denitrifiers are represented in all main phylogenetic groups.[3] Generally several species of bacteria are involved in the complete reduction of nitrate to N2, and more than one enzymatic pathway has been identified in the reduction process.[4]
Direct reduction from nitrate to ammonium, a process known as dissimilatory nitrate reduction to ammonium or DNRA,[5] is also possible for organisms that have the nrf-gene.[6] This is less common than denitrification in most ecosystems as a means of nitrate reduction. Other genes known in microorganisms which denitrify include nir (nitrite reductase) and nos (nitrous oxide reductase) among others;[7] organisms identified as having these genes include Alcaligenes faecalis, Alcaligenes xylosoxidans, many in the Pseudomonas genus, Bradyrhizobium japonicum, and Blastobacter denitrificans.[8]
Conditions required
Denitrification takes place under special conditions in both terrestrial and marine ecosystems.[9] In general, it occurs where oxygen, a more energetically favourable electron acceptor, is depleted, and bacteria respire nitrate as a substitute terminal electron acceptor. Due to the high concentration of oxygen in our atmosphere, denitrification only takes place in anoxic environments where oxygen consumption exceeds the oxygen supply and where sufficient quantities of nitrate are present. These environments may include certain soils[10] and groundwater,[11] wetlands, oil reservoirs,[12] poorly ventilated corners of the ocean, and in seafloor sediments.
Denitrification generally proceeds through some combination of the following intermediate forms:
- NO
3− → NO
2− → NO + N
2O → N
2 (g)
The complete denitrification process can be expressed as a redox reaction:
- 2 NO3− + 10 e− + 12 H+ → N2 + 6 H2O
This reaction shows a fractionation in isotope composition. Lighter isotopes of nitrogen are preferred in the reaction, leaving the heavier nitrogen isotopes in the residual matter. The process can cause delta-values of up to −40, where delta is a representation of the difference in isotopic composition . This can be used to identify denitrification processes in nature.
Use in wastewater treatment
Denitrification is commonly used to remove nitrogen from sewage and municipal wastewater. It is also an instrumental process in constructed wetlands[13] and riparian zones[14] for the prevention of groundwater pollution with nitrate resulting from excessive agricultural or residential fertilizer usage.[15] Wood chip bioreactors have been studied since the 2000s and are effective in removing nitrate from agricultural run off[16] and even manure.[17]
Reduction under anoxic conditions can also occur through process called anaerobic ammonium oxidation (anammox):[18]
- NH4+ + NO2− → N2 + 2 H2O
In some wastewater treatment plants, small amounts of methanol, ethanol, acetate, glycerin, or proprietary products are added to the wastewater to provide a carbon source for the denitrification bacteria.[19] Denitrification processes are also used in the treatment of industrial wastewater.[20]
See also
- Aerobic denitrification
- Anaerobic respiration
- Bioremediation
- Climate change
- Hypoxia (environmental)
- Nitrogen fixation
- Simultaneous nitrification-denitrification
References
- ↑ Carlson, C. A.; Ingraham, J. L. (1983). "Comparison of denitrification by Pseudomonas stutzeri, Pseudomonas aeruginosa, and Paracoccus denitrificans". Appl. Environ. Microbiol. 45: 1247–1253.
- ↑ Baalsrud, K., and K. S. Baalsrud. 1954. Studies on Thiobacillus denitrificans. Archives of Microbiology 20:34-62.
- ↑ Zumft, W (1997). "Cell biology and molecular basis of denitrification". Microbiol. Mol. Biol. Rev. 61: 533–616.
- ↑ Atlas, R.M., Barthas, R. Microbial Ecology: Fundamentals and Applications. 3rd Ed. Benjamin-Cummings Publishing. ISBN 0-8053-0653-6
- ↑ An, S., and W. S. Gardner. 2002. Dissimilatory nitrate reduction to ammonium (DNRA) as a nitrogen link, versus denitrification as a sink in a shallow estuary (Laguna Madre/Baffin Bay, Texas). Marine Ecology Progress Series 237:41-50.
- ↑ Spanning, R., M. Delgado, and D. Richardson. 2005. "It is possible to encounter DNRA when your source of carbon is a fermentable substrate, as glucose, so if you wanna avoid DNRA use a non fermentable substrate. The Nitrogen Cycle: Denitrification and its Relationship to N2 Fixation, p. 277-342."
- ↑ Zumft, W (1997). "Cell biology and molecular basis of denitrification". Microbiol. Mol. Biol. Rev. 61: 533–616.
- ↑ Liu, X.; Tiquia, S. M.; Holguin, G.; Wu, L.; Nold, S. C.; Devol, A. H.; Luo, K.; Palumbo, A. V.; Tiedje, J. M.; Zhou, J. (2003). "Molecular Diversity of Denitrifying Genes in Continental Margin Sediments within the Oxygen-Deficient Zone off the Pacific Coast of Mexico". Appl. Environ. Microbiol. 69: 3549–3560. doi:10.1128/aem.69.6.3549-3560.2003.
- ↑ Seitzinger, S.; Harrison, J. A.; Bohlke, J. K.; Bouwman, A. F.; Lowrance, R.; Peterson, B.; Tobias, C.; Drecht, G. V. (2006). "Denitrification Across Landscapes and Waterscapes: A Synthesis". Ecological Applications. 16: 2064–2090. doi:10.1890/1051-0761(2006)016[2064:dalawa]2.0.co;2.
- ↑ Scaglia, J.; Lensi, R.; Chalamet, A. (1985). "Relationship between photosynthesis and denitrification in planted soil". Plant and Soil. 84 (1): 37–43. doi:10.1007/BF02197865.
- ↑ Korom, Scott F. (1992). "Natural Denitrification in the Saturated Zone: A Review". Water Resources Research. 28 (6): 1657–1668. Bibcode:1992WRR....28.1657K. doi:10.1029/92WR00252.
- ↑ Cornish Shartau, S. L.; Yurkiw, M.; Lin, S.; Grigoryan, A. A.; Lambo, A.; Park, H. S.; Lomans, B. P.; Van Der Biezen, E.; Jetten, M. S. M.; Voordouw, G. (2010). "Ammonium Concentrations in Produced Waters from a Mesothermic Oil Field Subjected to Nitrate Injection Decrease through Formation of Denitrifying Biomass and Anammox Activity". Applied and Environmental Microbiology. 76 (15): 4977–4987. PMC 2916462
 . PMID 20562276. doi:10.1128/AEM.00596-10.
. PMID 20562276. doi:10.1128/AEM.00596-10. - ↑ Bachand, P. A. M., and A. J. Horne. 1999. Denitrification in constructed free-water surface wetlands: II. Effects of vegetation and temperature. Ecological Engineering 14:17-32.
- ↑ Martin, T. L., N. K. Kaushik, J. T. Trevors, and H. R. Whiteley. 1999. Review: Denitrification in temperate climate riparian zones. Water, Air, and Soil Pollution 111:171-186.
- ↑ Mulvaney, R. L., S. A. Khan, and C. S. Mulvaney. 1997. Nitrogen fertilizers promote denitrification. Biology and Fertility of Soils 24:211-220.
- ↑ Ghane E, Fausey NR, Brown LC. "Modeling nitrate removal in a denitrification bed. Water Res. 2015 Jan 30;71C:294-305. doi:10.1016/j.watres.2014.10.039. PMID 25638338 (subscription required)
- ↑ Carney KN1, Rodgers M, Lawlor PG, Zhan X. "Treatment of separated piggery anaerobic digestate liquid using woodchip biofilters." Environ Technology. 2013 Mar-Apr;34(5-8):663-70. doi:10.1080/09593330.2012.710408 PMID 23837316 (subscription required)
- ↑ Dalsgaard, T.; Thamdrup, B.; Canfield, D. E. (2005). "Anaerobic ammonium oxidation (anammox) in the marine environment". Research in Microbiology. 156: 457–464. doi:10.1016/j.resmic.2005.01.011.
- ↑ Chen, K.-C.; Lin, Y.-F. (1993). "The relationship between denitrifying bacteria and methanogenic bacteria in a mixed culture system of acclimated sludges". Water Research. 27: 1749–1759. doi:10.1016/0043-1354(93)90113-v.
- ↑ Constantin, H.; Fick, M. (1997). "Influence of C-sources on the denitrification rate of a high-nitrate concentrated industrial wastewater". Water Research. 31: 583–589. doi:10.1016/s0043-1354(96)00268-0.
Literature
- Atlas, R.M., Barthas, R. Microbial Ecology: Fundamentals and Applications. 3rd Ed. Benjamin-Cummings Publishing. ISBN 0-8053-0653-6
- "Cell biology and molecular basis of denitrification". Microbiol. Mol. Biol. Rev. 61 (4): 533–616. December 1997. PMC 232623
 . PMID 9409151. PDF
. PMID 9409151. PDF