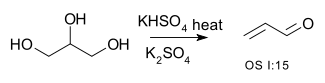Dehydration reaction
In chemistry and the biological sciences, a dehydration reaction is a chemical reaction that involves the loss of a water molecule from the reacting molecule. Dehydration reactions are a subset of condensation reactions. Because the hydroxyl group (–OH) is a poor leaving group, having a Brønsted acid catalyst often helps by protonating the hydroxyl group to give the better leaving group, –OH2+. The reverse of a dehydration reaction is a hydration reaction. Common dehydrating agents used in organic synthesis include concentrated sulfuric acid, concentrated phosphoric acid, hot aluminium oxide and hot ceramic.
Dehydration reactions and dehydration synthesis have the same meaning, and are often used interchangeably. Two monosaccharides, such as glucose and fructose, can be joined together (to form sucrose) using dehydration synthesis. The new molecule, consisting of two monosaccharides, is called a disaccharide.
The process of hydrolysis is the reverse reaction, meaning that the water is recombined with the two hydroxyl groups and the disaccharide reverts to being monosaccharides.
In the related condensation reaction water is released from two different reactants.
Dehydration reactions
In organic synthesis, there are many examples of dehydration reaction, for example dehydration of alcohols or sugars.
| Dehydration reactions | ||
| Reaction | Equation | |
|---|---|---|
| Conversion of alcohols to ethers | 2 R–OH → R–O–R + H2O | |
| Conversion of alcohols to alkenes | R–CH2−CHOH–R → R–CH=CH–R + H2O | for example the conversion of glycerol to acrolein:[1]
or the dehydration of 2-methyl-1-cyclohexanol to (mainly) 1-methylcyclohexene [2] |
| Conversion of carboxylic acids to acid anhydrides | 2 RCOOH → (RCO)2O + H2O | |
| Conversion of amides to nitriles | RCONH2 → R–CN + H2O | |
| Dienol benzene rearrangement |  [3][4] [3][4] | |
Some dehydration reactions can be mechanistically complex, for instance the reaction of a sugar (sucrose) with concentrated sulfuric acid:[5] to form carbon as a graphitic foam involves formation of carbon-carbon bonds.[6] The reaction is driven by the strongly exothermic reaction as sulfuric acid reacts with water, which produces dangerous sulfuric-acid containing steam, therefore the experiment should only be performed in a fume-hood or well ventilated area.
See also
References
- ↑ H. Adkins; W. H. Hartung (1926). "Acrolein". Org. Synth. 6: 1. doi:10.15227/orgsyn.006.0001.; Coll. Vol., 1, p. 15
- ↑ J. Brent Friesen; Robert Schretzman (2011). "Dehydration of 2-Methyl-1-cyclohexanol: New Findings from a Popular Undergraduate Laboratory Experiment". J. Chem. Educ. 88 (8): 1141–1147. doi:10.1021/ed900049b.
- ↑ H. Plieninger; Gunda Keilich (1956). "Die Dienol-Benzol-Umlagerung" [The dienol-benzene rearrangement]. Angew. Chem. (in German). 68 (19): 618. doi:10.1002/ange.19560681914.
- ↑ Margaret Jevnik Gentles; Jane B. Moss; Hershel L. Herzog; E. B. Hershberg (1958). "The Dienol-Benzene Rearrangement. Some Chemistry of 1,4-Androstadiene-3,17-dione". J. Am. Chem. Soc. 80 (14): 3702–3705. doi:10.1021/ja01547a058.
- ↑ youtube clip reaction sugar with sulfuric acid
- ↑ http://www.exo.net/~pauld/activities/astronomy/transitvenus/sugarsulfuricacid.htm