Evolution of mammalian auditory ossicles
| Part of a series on |
| Paleontology |
|---|
 |
|
Organs and processes |
|
History of paleontology |
|
Branches of paleontology |
|
Paleontology Portal Category |
The evolution of mammalian auditory ossicles is one of the most well-documented[1] and important[2] evolutionary events, demonstrating both numerous transitional forms as well as an excellent example of exaptation, the re-purposing of existing structures during evolution.
In reptiles, the eardrum is connected to the inner ear via a single bone, the columella, while the upper and lower jaws contain several bones not found in mammals. Over the course of the evolution of mammals, one lower and one upper jaw bone (the articular and quadrate) lost their purpose in the jaw joint and were put to new use in the middle ear, connecting to the existing stapes bone and forming a chain of three bones (collectively called the ossicles) which transmit sounds more efficiently and allow more acute hearing. In mammals, these three bones are known as the malleus, incus, and stapes (hammer, anvil, and stirrup respectively). Mammals and birds also differ from other vertebrates by having evolved a cochlea.
The evidence that the malleus and incus are homologous to the reptilian articular and quadrate was originally embryological, and since this discovery an abundance of transitional fossils has both supported the conclusion and given a detailed history of the transition.[3] The evolution of the stapes (from the hyomandibula) was an earlier and distinct event.[4][5]
Reichert–Gaupp theory
Following on the ideas of Étienne Geoffroy Saint-Hilaire (1818), and studies by Johann Friedrich Meckel the Younger (1820), Carl Gustav Carus (1818), Martin Rathke (1825), and Karl Ernst von Baer (1828),[6] the relationship between the reptilian jaw bones and mammalian middle-ear bones was first established on the basis of embryology and comparative anatomy by Karl Bogislaus Reichert (in 1837, before the publication of On the Origin of Species in 1859) and advanced by Ernst Gaupp [7] and this is known as the Reichert–Gaupp theory.[8][9]
In the course of the development of the embryo, the incus and malleus arise from the same first pharyngeal arch as the mandible and maxilla, and are served by mandibular and maxillary division of the trigeminal nerve.[10]
...the discovery that the mammalian malleus and incus were actually homologues of visceral elements of the "reptilian" jaw articulation ... ranks as one of the milestones in the history of comparative biology.[11]
... it is one of the triumphs of the long series of researches on the extinct Theromorph reptiles, begun by Owen (1845), and continued by Seeley, Broom, and Watson, to have revealed the intermediate steps by which the change may have occurred from an inner quadrate to an outer squamosal articulation ...[12]
Yet the transition between the "reptilian" jaw and the "mammalian" middle ear was not bridged in the fossil record until the 1950s[13] with the elaboration of such fossils as the now-famous Morganucodon.[14]
There are also more recent studies in the genetic basis for the development of the ossicles from the embryonic arch,[15] and relating this to evolutionary history.[16]
"Bapx1, also known as Nkx3.2, is the vertebrate homologue of the Drosophila gene Bagpipe. A member of the NK2 class of homeobox genes ...",[17] this gene is implicated in the change from the jaw bones of non-mammals to the ossicles of mammals.[18][19] Others are Dlx genes, Prx genes, and Wnt genes.[20]
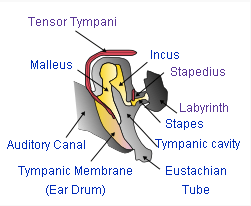
Definitive mammalian middle ear
The mammalian middle ear contains three tiny bones known as the ossicles: malleus, incus, and stapes. The ossicles are a complex system of levers whose functions include: reducing the amplitude of the vibrations; increasing the mechanical force of vibrations; and thus improving the efficient transmission of sound energy from the eardrum to the inner ear structures. The ossicles act as the mechanical analog of an electrical transformer, matching the mechanical impedance of vibrations in air to vibrations in the liquid of the cochlea. The net effect of this impedance matching is to greatly increase the overall sensitivity and upper frequency limits of mammalian hearing, as compared to reptilian hearing. The details of these structures and their effects vary noticeably between different mammal species, even when the species are as closely related as humans and chimpanzees.[21]
Evolutionary history
Definition of "mammal"
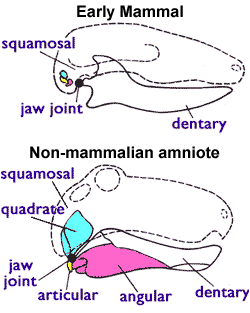
Living mammal species can be identified by the presence in females of mammary glands which produce milk. Other features are required when classifying fossils, since mammary glands and other soft-tissue features are not visible in fossils. Paleontologists therefore use a distinguishing feature that is shared by all living mammals (including monotremes), but is not present in any of the early Triassic therapsids ("mammal-like reptiles"): mammals use two bones for hearing that all other amniotes use for eating. The earliest amniotes had a jaw joint composed of the articular (a small bone at the back of the lower jaw) and the quadrate (a small bone at the back of the upper jaw). All non-mammalian amniotes use this system including lizards, crocodilians, dinosaurs (and their descendants the birds) and therapsids; so the only ossicle in their middle ears is the stapes. But mammals have a different jaw joint, composed only of the dentary (the lower jaw bone which carries the teeth) and the squamosal (another small skull bone). In mammals, the quadrate and articular bones have evolved into the incus and malleus bones in the middle ear.[22][23]
Summary of the fossil evidence
Here is a very simplified "family tree" of the various lineages involved:
--Tetrapods------ | (literally "4 legged"; the earliest breathed via gills) | +-- Amphibians ---------------------------------------------- | `--------Reptiliomorphs----- | ("reptile-like" amphibians) | `--Amniotes------ | +--Sauropsids ("lizard faces")--------------- | (lizards, crocodilians, dinosaurs, birds | Testudines; and some extinct groups) | `--Synapsids------ | `--Pelycosaurs*---- | `--Therapsids----- | `--Mammals---------------
The first fully terrestrial vertebrates were amniotes - their eggs had internal membranes which allowed the developing embryo to breathe but kept water in. This allowed amniotes to lay eggs on dry land, while amphibians generally need to lay their eggs in water. The first amniotes apparently arose in the late Carboniferous from the ancestral reptiliomorphs (a group of amphibians whose only living descendants are amniotes). Within a few million years two important amniote lineages became distinct: mammals' synapsid ancestors and the sauropsids, from which lizards, snakes, crocodilians, dinosaurs and birds are descended.[24]
The earliest known fossils of all these groups date from about 320 to 315M years ago. Unfortunately it is difficult to be sure about when each of them evolved, since vertebrate fossils from the late Carboniferous are very rare, and therefore the actual first occurrences of each of these types of animal might have been considerably earlier.[23][25]
The pattern in most of the following sections is that each successive more "advanced" group started with the more "primitive" jaws and ears of its predecessors, then developed more mammal-like jaws and ears, and so on. The evolution of mammalian jaw joints and ears did not proceed neatly in lockstep with the evolution of other mammalian features. In other words, jaw joints and ears do not define any except the last of the various stages into which paleontologists divide the evolution towards the mammalian anatomy.
Early tetrapod and amniote ears
In modern amniotes (including mammals), the middle ear collects airborne sounds through an ear drum and transmits the vibrations to the inner ear via thin cartilaginous and ossified structures, which usually include the stapes (a stirrup-shaped auditory ossicle). But the earliest tetrapods, amphibians and amniotes probably did not have ear drums. In fact ear drums apparently evolved independently three to six times, in: stegocephalians (very primitive amphibians); in anurans (the amphibian group that includes frogs and toads); in synapsids (mammals and their extinct relatives), in diapsids (the most important sauropsid group, including lizards, crocodiles, dinosaurs and birds); perhaps separately in anapsids (turtles and their extinct relatives), if turtles are not modified diapsids; probably in seymouriamorphs (a group of reptiliomorphs); and possibly in some temnospondyls (primitive amphibians).[26][27] In all basal members of the 3 major clades of amniotes (synapsids, eureptiles, and parareptiles) the stapes bones are relatively massive props that support the braincase, and this function prevents them from being used as part of the hearing system. But there is increasing evidence that synapsids, eureptiles and parareptiles developed eardrums connected to the inner ear by stapes during the Permian.[28]
Early therapsid jaws and ears
The jaws of early synapsids, including the ancestors of mammals, were similar to those of other tetrapods of the time, with a lower jaw consisting of a tooth-bearing dentary bone and several smaller posterior bones. The jaw joint consisted of the articular bone in the lower jaw and the quadrate in the upper jaw. The early pelycosaurs (late Carboniferous and early Permian) most probably did not have tympanic membranes (external eardrums), and their massive stapes bones supported the braincase, with the lower ends resting on the quadrates. But their descendants the therapsids (including mammals' ancestors) probably did have tympanic membranes and these probably were in contact with the quadrate bones; and the stapes bones were still in contact with the quadrates but functioned as auditory ossicles rather than braincase supports; so the therapsids' quadrates had a dual function, as part of the jaw joint and as parts of the hearing system.[29][30]
Twin-jointed jaws
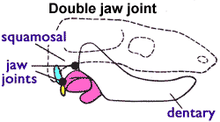
During the Permian and early Triassic the dentary of therapsids, including the ancestors of mammals, continually enlarged while other jaw bones were reduced.[31][31] Eventually, the dentary was able to make contact with the squamosal, a bone in the upper jaw located anterior to the quadrate, allowing two simultaneous jaw joints[32] - an anterior "mammalian" joint between the dentary and squamosal and a posterior "reptilian" joint between the quadrate and articular. This "twin-jointed jaw" can be seen in late cynodonts and early mammaliforms.[33] Morganucodon is one of the first discovered and most thoroughly studied of the mammaliforms, since an unusually large number of morganucodont fossils have been found, and
Morganucodon is an almost perfect intermediate in this respect (the "twin-jointed jaw") between the higher mammal-like reptiles on the one hand and the typical mammals on the other.[34]
(note: "mammal-like reptiles" is an obsolete term for the therapsids)
Mammal-like jaws and ears
As the dentary continued to enlarge during the Triassic, the older quadrate-articular joint fell out of use. Some of the bones were lost, but the quadrate (which is directly connected to the stapes), the articular (connected to the quadrate) and the angular (connected to the articular) became free-floating and associated with the stapes. This occurred at least twice in the mammaliformes ("almost-mammals"). The Multituberculates, which lived from about 160M years ago (mid-Jurassic) to about 35M years ago (early Oligocene) had jaw joints that consisted of only the dentary and squamosal bones, and the quadrate and articular bones were part of the middle ear; but other features of their teeth, jaws and skulls are significantly different from those of mammals.[23][35]
In the lineage most closely related to mammals, the jaws of Hadrocodium (about 195M years ago in the very early Jurassic) suggest that it or a very close ancestor may have been the first to have a nearly fully mammalian middle ear: it lacks the trough at the rear of the lower jaw, over which the eardrum stretched in therapsids and earlier mammaliformes, and the absence of this trough which suggests that Hadrocodium’s ear was part of the cranium, as it is in mammals, and hence that the former articular and quadrate had migrated to the middle ear and become the malleus and incus; but Hadrocodium’s dentary has a "bay" at the rear which mammals lack, a hint that its dentary bone retained the same shape that it would have had if the articular and quadrate had remained part of the jaw joint.[36]
It has been suggested that a relatively large trough in the jaw bone of the early Cretaceous monotreme Teinolophos provides evidence of a pre-mammalian jaw joint, because therapsids and many mammaliforms had such troughs, in which the articular and angular bones "docked", and therefore that Teinolophos had a pre-mammalian middle ear; and therefore that the mammalian middle ear ossicles evolved independently in monotremes and in other mammals.[37] But a more recent analysis of Teinolophos concluded that the animal was a full-fledged platypus and the trough was a channel for the large number of nerves that collect signals from the electrical and vibration sensors in the bill (this is a signature feature of the platypus within monotremes), and therefore that the trough is not evidence that Teinolophos had a pre-mammalian jaw joint and a pre-mammalian middle ear.[38] Ironically Rich and Vickers-Rich were among the authors of the 2005 paper on which they later cast doubt.
A recently discovered intermediate form is the primitive mammal Yanoconodon, from 125 million years ago in the Mesozoic, in which the ossicles have separated from the jaw and serve the hearing function in the middle ear, yet maintain a slender connection to the jaw via the ossified Meckel's cartilage, which in more advanced mammals dissolves during development.[39][40]
How these changes affected hearing
The frequency range and sensitivity of the ear is dependent upon the shape and arrangement of the middle-ear bones. In early synapsids such as the pelycosaurs, the quadrate and articular had to function as the jaw joint, and this severely limited how far these bones could be modified to alter the frequency range of the ear. But once these bones were no longer involved in the jaw joint, variations which affected hearing would not also affect jaw joint function, and this allowed unconstrained evolution of the mammalian hearing apparatus.[41] By the Jurassic, the typical mammalian ear had evolved, in which the angular had become the tympanic annula (a bony support for the tympanic membrane), while the articular and quadrate had become the malleus and incus, respectively, connected in series with the stapes. This series of three bones acts as an impedance matching system to improve sound transmission and allow enhanced hearing.[42]
The transition between these two states is one of the most well-documented[43] and supported in all of evolution, and newly discovered fossils from this transitional period have recently improved our understanding of this transition. But they also suggest that it was not a simple linear process from the early therapsid jaw (quadrate-articular joint) and middle ear (with stapes as the only ossicles) to the modern mammalian anatomy.[30]
Natural selection
It has been suggested that natural selection could be a factor in the preservation of the structure of the middle ear in mammals.[1][30] Many of the earliest mammals were quite small, and the dentition indicates that they were insectivorous. If they were "warm-blooded" (endothermic), like modern mammals, then they could have been nocturnal. This fits with the popular image of small, nocturnal insectivorous mammals surviving in niches not accessible to the large, dominant contemporary dinosaurs. The enhanced hearing, particularly in the higher frequencies, would be helpful for nocturnal animals, in particular for detecting insects.[44][45] This scenario is consistent with selective advantage being a contributory factor to the transition.
Summary
By extrapolating the developmental morphogenesis of genetic studies into the early mammal fossil record, evolution of the middle ear in early mammals provides an integrated case study of how development has impacted, mechanistically, the transformation of a major structural complex in evolution.— Zhe-Xi Luo, Developmental Patterns in Mesozoic Evolution of Mammal Ears
While the stapes is present in many types of tetrapods, the addition of the incus and malleus (also known as quadrate and articular) in the middle ear is a signature feature of mammals, distinguishing them from reptiles and all other vertebrates. They therefore have the appearance of representing a discontinuity in the tree of life. But in the early 19th century, it was hypothesized that these bones are not a total novelty, but are the equivalents of two bones which non-mammals have in their jaws. This hypothesis made sense, not only of the existence of these middle-ear bones, but also of certain other features of the anatomy, such as the paths taken by nerves in the head.
As evolutionary biology began to be expanded upon, this relationship became treated as one of common descent. For the evolutionary explanation to make sense, it seemed to demand that there would be a transition in function between being part of the feeding mechanism in the joint of the jaw and serving only in hearing; and this would mean that somehow there had to be an intermediate connecting these two quite different functions. With the discovery of Morganucodon and other[33] fossils, there were concrete examples of this. There was a double jaw joint: the "older reptilian", as well as the "newer mammalian", in the same animal. This meant a confirmation of the pattern of inference from comparative anatomy to evolutionary biology.
The earliest mammals were generally small animals, probably nocturnal insectivores. This suggests a plausible evolutionary mechanism driving the change; for with these small bones in the middle ear, a mammal has extended its range of hearing for higher-pitched sounds which would improve the detection of insects in the dark.[46] Natural selection would account for the success of this feature. There is still one more connection with another part of biology: genetics suggests a mechanism for this transition, the kind of major change of function seen elsewhere in the world of life being studied by evolutionary developmental biology.[47]
See also
References
- Maier, Wolfgang; Ruf, Irina (February 2016). "Evolution of the mammalian middle ear: a historical review". Journal of Anatomy. 228 (2): 270–283. PMC 4718169
 . PMID 26397963. doi:10.1111/joa.12379.
. PMID 26397963. doi:10.1111/joa.12379.
- 1 2 Allin EF (December 1975). "Evolution of the mammalian middle ear". Journal of Morphology. 147 (4): 403–437. PMID 1202224. doi:10.1002/jmor.1051470404.
- ↑ Meier & Ruf (2016), page 270, Introduction, "The study of the mammalian middle ear has been one of the central themes of vertebrate morphological research of the last 200 years."
- ↑ Bowler, Peter J. (1996). "Chapter 6: The Origin of Birds and Mammals". Life's splendid drama: evolutionary biology and the reconstruction of life's ancestry, 1860-1940. Chicago: University of Chicago Press. ISBN 0-226-06921-4.
- ↑ Janvier, Philippe (2002). Early vertebrates. Oxford Monographs on Geology and Geophysics, 33. Oxford: Clarendon Press. p. 56. ISBN 978-0-19-852646-9.
- ↑ The Shoulder Bone’s Connected to the Ear Bone… Carl Zimmer blog The Loom at Discover magazine for 2008 October 15.
- ↑ Maier & Ruf (2016) p. 272, The Reichert-Gaupp theory.
- ↑ Ernst Gaupp. "Zur Entwickelungsgeschichte und vergleichen Morphologie des Schädels von Echidna aculeata var. ehenden typical" [On the developmental history and comparative morphology of the skull of Echidna aculeata var. typical]. Richard Semon Fortschungsreisen (in German). 3: 539–788.
- ↑ Masaki Takechi; Shigeru Kuratani (2010). "History of Studies on Mammalian Middle Ear Evolution: A Comparative Morphological and Developmental Biology Perspective". Journal of Experimental Zoology: Part B Molecular and Developmental Evolution. 314B (6): 417–433. doi:10.1002/jez.b.21347.
- ↑ Toby A. Appel (1987). The Cuvier–Geoffroy Debate: French Biology in the Decades before Darwin. New York and Oxford: Oxford University Press. pp. 206–207. ISBN 0-19-504138-0.
- ↑ Gilbert, Scott F. (2003). Developmental biology (7th ed.). Sunderland, Mass: Sinauer Associates. p. 435. ISBN 0-87893-258-5.
- ↑ Novacek MJ (1993). Hall BK, Hanken J, eds. The Skull. Chicago: University of Chicago Press. pp. 438–545. ISBN 0-226-31568-1. Novacek references these early works: Johann Friedrich Meckel (1820). Handbuch der Menschlichen Anatomie [Handbook of Human Anatomy] (in German). Halle. – Reichert KB (1837). "Ueber die Visceralbogen der Wirbelthiere im Allegemeinen und deren Metamorphosen bei den Vögln und Säugethieren" [On the visceral arches of the vertebrates in general and their metamorphoses among the birds and mammals]. Archiv für Anatomie, Physiologie, und wissenschaftliche Medizin (in German). Leipzig: 120–122. – Gaupp E (1913). "Die Reichertsche Theorie (Hammer-, Amboss- und Kieferfrage)" [The Reichert theory (question of the hammer, anvil and stirrup)]. Archiv für Anatomie und Entwicklungsgeschichte (in German): 1–416.
- ↑ Goodrich ES (1958) [1934]. Studies on the Structure and Development of Vertebrates. Dover. p. 474.
- ↑ AW Crompton; FA Jenkins, Jr (1973). "Mammals from Reptiles: A Review of Mammalian Origins". Annual Review of Earth and Planetary Sciences. 1: 131–155. doi:10.1146/annurev.ea.01.050173.001023.
- ↑ Walter Georg Kühne (1958). "Rhaetische Triconodonten aus Glamorgan, ihre Stellung zwischen den Klassen Reptilia und Mammalia und ihre Bedeutung für die REICHART'sche Theorie" [Rhaetic triconodonts from Glamorgen, their place between the Reptilia and Mammalia classes and their meaning for the Reichart theory]. Palaeontologische Zeitschrift (in German). 32 (3/4): 197–235. doi:10.1007/BF02989032.
- ↑ Mallo M (March 2001). "Formation of the middle ear: recent progress on the developmental and molecular mechanisms". Developmental Biology. 231 (2): 410–419. PMID 11237469. doi:10.1006/dbio.2001.0154.
- ↑ Raff RA (December 2007). "Written in stone: fossils, genes and evo-devo". Nature Reviews Genetics. 8 (12): 911–920. PMID 18007648. doi:10.1038/nrg2225.
- ↑ Wilson J, Tucker AS (February 2004). "Fgf and Bmp signals repress the expression of Bapx1 in the mandibular mesenchyme and control the position of the developing jaw joint". Developmental Biology. 266 (1): 138–150. PMID 14729484. doi:10.1016/j.ydbio.2003.10.012.
- ↑ Tucker AS, Watson RP, Lettice LA, Yamada G, Hill RE (March 2004). "Bapx1 regulates patterning in the middle ear: altered regulatory role in the transition from the proximal jaw during vertebrate evolution". Development. 131 (6): 1235–1245. PMID 14973294. doi:10.1242/dev.01017.
- ↑ A survey of the genes involved in the development of the vertebrate middle ear is given in Susan Caroline Chapman (January 1, 2011). "Can you hear me now? Understanding vertebrate middle ear development". Frontiers in Bioscience. 16 (2): 1675–1693. PMC 3065862
 . PMID 21196256. doi:10.2741/3813.
. PMID 21196256. doi:10.2741/3813. - ↑ Sienknecht UJ (July 2013). "Developmental origin and fate of middle ear structures". Hearing Research. 301 (MEMRO [Middle Ear Mechanics in Research and Otology, international symposium for the year] 2012 – Middle-Ear Bridge between Science and Otology): 19–26. doi:10.1016/j.heares.2013.01.019.
- ↑ Masali M (October 1992). "The ear ossicles and the evolution of the primate ear: A biomechanical approach". Human Evolution. Springer Netherlands. 7 (4): 1–5. doi:10.1007/BF02436407.
- ↑ White T. "Unit 430: Mammalia: Overview". PALAEOS: The Trace of Life on Earth. palaeos.com. Archived from the original on June 15, 2008. Retrieved 2008-07-21.
- 1 2 3 Cowen, Richard (2000). History of life. Oxford: Blackwell Science. p. 432. ISBN 0-632-04444-6.
- ↑ White T. "Amniota". PALAEOS: The Trace of Life on Earth. palaeos.com. Retrieved 2008-07-21.
- ↑ White T. "Synapsida: Varanopseidae". PALAEOS: The Trace of Life on Earth. palaeos.com. Archived from the original on May 11, 2008. Retrieved 2008-07-21.
- ↑ Laurin M (January–March 1998). "The importance of global parsimony and historical bias in understanding tetrapod evolution. Part I. Systematics, middle ear evolution and jaw suspension". Annales des Sciences Naturelles - Zoologie et Biologie Animale. 19 (1): 1–42. doi:10.1016/S0003-4339(98)80132-9.
- ↑ Laurin M. "Hearing in Stegocephalians". Tree of Life. Tree of Life Project. Retrieved 2008-07-21.
- ↑ Müller J, Tsuji LA (2007). Clack, Jenny, ed. "Impedance-matching hearing in Paleozoic reptiles: evidence of advanced sensory perception at an early stage of amniote evolution". PLoS ONE. 2 (9): e889. PMC 1964539
 . PMID 17849018. doi:10.1371/journal.pone.0000889.
. PMID 17849018. doi:10.1371/journal.pone.0000889. - ↑ Fay, Richard R.; Manley, Geoffrey A.; Popper, Arthur N. (2004). Evolution of the vertebrate auditory system. Berlin: Springer. ISBN 0-387-21089-X.
- 1 2 3 Luo ZX (December 2007). "Transformation and diversification in early mammal evolution" (PDF). Nature. 450 (7172): 1011–1019. PMID 18075580. doi:10.1038/nature06277. Archived from the original (PDF) on November 27, 2012.
- 1 2 Sidor CA (July 2001). "Simplification as a trend in synapsid cranial evolution". Evolution. 55 (7): 1419–42. PMID 11525465. doi:10.1554/0014-3820(2001)055[1419:saatis]2.0.co;2.
- ↑ Page 229, Michael J. Benton, Vertebrate Palaeontology: Biology and evolution, Unwin Hyman, 1990 ISBN 0-04-566001-8
- 1 2 Page 228 of Edward H. Colbert and Michael Morales, Evolution of the Vertebrates: A History of the Backboned Animals Through Time, Wiley-Liss, 4th edition, 1991 ISBN 0-471-85074-8
- ↑ Kermack KA, Mussett F, Rigney HW (January 1981). "The skull of Morganucodon". Zoological Journal of the Linnean Society. 71 (1): 1–158. doi:10.1111/j.1096-3642.1981.tb01127.x.
- ↑ White T. "Mammaliformes". PALAEOS: The Trace of Life on Earth. palaeos.com. Archived from the original on June 4, 2008. Retrieved 2008-07-21.
- ↑ White T. "Symmetrodonta". PALAEOS: The Trace of Life on Earth. palaeos.com. Archived from the original on July 3, 2008. Retrieved 2008-07-21.
- ↑ Rich TH, Hopson JA, Musser AM, Flannery TF, Vickers-Rich P (February 2005). "Independent origins of middle ear bones in monotremes and therians". Science. 307 (5711): 910–914. PMID 15705848. doi:10.1126/science.1105717.
- ↑ Rowe T, Rich TH, Vickers-Rich P, Springer M, Woodburne MO (January 2008). "The oldest platypus and its bearing on divergence timing of the platypus and echidna clades". Proceedings of the National Academy of Sciences of the United States of America. 105 (4): 1238–1242. PMC 2234122
 . PMID 18216270. doi:10.1073/pnas.0706385105.
. PMID 18216270. doi:10.1073/pnas.0706385105. - ↑ PZ Myers (March 16, 2007). "Yanoconodon, a transitional fossil". Pharyngula: Evolution, development, and random biological ejaculations from a godless liberal.
- ↑ Ramíres-Chaves, Héctor E.; Weisbecker, Vera; Wroe, Stephen; Phillips, Matthew J. (2016). "Resolving the evolution of the mammalian middle ear using Baysian inference". Frontiers in Zoology. 13 (39). doi:10.1186/s12983-016-0171-z.
- ↑ Lombard RE, Hetherington TE (1993). "Structural Basis of Hearing and Sound Transmission". In Hall BK, Hanken J. The Skull (volume 3 ed.). Chicago: University of Chicago Press. pp. 241–302. ISBN 0-226-31571-1.
- ↑ Köppl C (11 August 2009). "Evolution of sound localization in land vertebrates". Current Biology. 19 (15): R635–R639. PMID 19674542. doi:10.1016/j.cub.2009.05.035.
- ↑ Cuffey CA. "References". The Fossil Record: Evolution or "Scientific Creation". GCSSEPM Foundation. Archived from the original on 2008-05-21. Retrieved 2008-07-21.
- ↑ Geoffrey A. Manley (2012). "Evolutionary Paths to Mammalian Cochleae". JARO - Journal of the Association for Research in Otolaryngology. 13 (6): 733–743. doi:10.1007/s10162-012-0349-9.
- ↑ Urban, Daniel J.; Anthwal, Neal; Luo, Zhe-Xi; Maier, Jennifer A.; Sadier, Alexa; Tucker, Abigail S. (2017). "A new developmental mechanism for the separation of the mammalian middle ear ossicles from the jaw". Proceeding of the Royal Society B. 284 (1848): 20162416. doi:10.1098/rspb.2016.2416.
- ↑ Biello D (2007-03-14). "From Jaw to Ear: Transition Fossil Reveals Ear Evolution in Action". Scientific American. Retrieved 2009-06-17.
Now hear this: early mammal fossil shows how sensitive ear bones evolved
- ↑ Maier & Ruf (2006), p. 279, Developmental biology, developmental genetics, and phylogeny
Further reading
- Allin EF, Hopson JA (1992). "Chapter 28: Evolution of the Auditory System in Synapsida ("Mammal-Like Reptiles" and Primitive Mammals) as Seen in the Fossil Record". In Popper AN, Webster DB, Fay RR. The Evolutionary biology of hearing. Berlin: Springer-Verlag. pp. 587–614. ISBN 0-387-97588-8.
- Anthwal, Neal; Joshi, Leena; Tucker, Abigail S (2012). "Evolution of the mammalian middle ear and jaw: adaptations and novel structures". Journal of Anatomy. 222 (1): 1–96. PMC 3552421
 . PMID 22686855. doi:10.1111/j.1469-7580.2012.01526.x.
. PMID 22686855. doi:10.1111/j.1469-7580.2012.01526.x. - Arthur, Wallace (2011). "10.3 Compound Repatterning at a Single Level of Organisation". Evolution: A developmental approach. Oxford: Wiley-Blackwell. pp. 151–155. ISBN 978-1-4051-8658-2.
- Asher, Robert J. (2012). Evolution and belief: confessions of a religious paleontologist. Cambridge & New York: Cambridge University Press. pp. 93–110, 196–200. ISBN 9780521193832.
- Gould SJ (1993). "Chapter 6: An Earful of Jaw". Eight Little Piggies: reflections in natural history. New York: Norton. ISBN 0-393-03416-X.
- Hopson JA (January 1987). "The mammal-like reptiles: a study of transitional fossils". The American Biology Teacher. 49 (1): 16–26. JSTOR 4448410. doi:10.2307/4448410.
- Kielan-Jaworowska, Z (2013). "5. Origins of Mammals and the Earliest Representatives of Mammaliforms and Mammals". In Pursuit of Early Mammals. Life of the Past. Bloomington, Indiana: Indiana University Press. pp. 73–96. ISBN 978-0-253-00824-4. especially pages 85–96
- Luo ZX, Kielan-Jaworowska Z, Cifelli RL (2004). "Chapter 3: Origin of mammals". Mammals from the age of dinosaurs: origins, evolution, and structure. New York: Columbia University Press. ISBN 0-231-11918-6.
- Luo, Zhe-Xi (2011). "Developmental Patterns in Mesozoic Evolution of Mammal Ears". Annual Review of Ecology, Evolution, and Systematics. 42: 355–380. doi:10.1146/annurev-ecolsys-032511-142302.
- Manley GA, Sienknecht UJ (2013). "Chapter 2: The Evolution and Development of Middle Ears in Land Vertebrates". In Puria S, Fay RR, Popper AN. The Middle Ear: Science, Otosurgery, and Technology. Springer Handbook of Auditory Research. 46. New York: Springer. pp. 7–30. ISBN 978-1-4614-6590-4. doi:10.1007/978-1-4614-6591-1_2.
- Meng J, Zheng XT, Wang XL (2016). "Ear Ossicle Morphology of the Jurassic Euharamiyidan Arboroharamiya and Evolution of Mammalian Middle Ear". Journal of Morphology. doi:10.1002/jmor.20565. contains wide bibliography of scientific literature up to 2016
- Rosowski JJ (1992). "Chapter 29: Hearing in Transitional Mammals: Predictions from the Middle-Ear Anatomy and Hearing Capabilities of Extant Mammals". In Popper AN, Webster DB, Fay RR. The Evolutionary biology of hearing. Berlin: Springer-Verlag. pp. 615–632. ISBN 0-387-97588-8.
- Rougier GW, White JR (2006). "Chapter 6: Major Changes in the Ear Region and Basicranium of Early Mammals". In Carrano MT, Gaudin TJ, Blob RW, Wible JR. Amniote paleobiology: perspectives on the evolution of mammals, birds, and reptiles: a volume honoring James Allen Hopson. Chicago: University of Chicago Press. pp. 269–311. ISBN 0-226-09477-4.
- Shubin N (2008). "Chapter 10: Ears". Your inner fish: a journey into the 3.5-billion-year history of the human body. New York: Pantheon Books. ISBN 0-375-42447-4.
- Tucker, Abigail S. (5 February 2017). "Major evolutionary transitions and innovations: the tympanic middle ear" (PDF). Philosophical Transactions of the Royal Society B. 372 (1713): 20150483. doi:10.1098/rstb.2015.0483.
External links
- Theobald D (2004). "29+ Evidences for Macroevolution: Part 1, Example 2: reptile-mammals". TalkOrigins. Retrieved 2009-06-17.
- "The Evolution of Hearing from Amphibians to Mammals". 2004. Retrieved 2016-03-26.
- Cuffey CA (2001). "The Fossil Record: Evolution or "Scientific Creation": Mammal-Like Reptiles". GCSSEPM Foundation. Archived from the original on May 1, 2009. Retrieved 2009-06-17.
- Matzke N (2005). "The testimony of Kevin Padian in Kitzmiller v. Dover". sciohost.org. Retrieved 2009-06-17.
Based on testimony by Kevin Padian in the case of Kitzmiller v. Dover
- Your Inner Fish : We Hear With the Bones That Reptiles Eat With