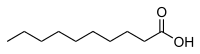
Decanoic acid
- For the term capric as it related to music see Capriccio (music)
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Decanoic acid | |
| Other names
Capric acid,[1] n-Capric acid, n-Decanoic acid, Decylic acid, n-Decylic acid, C10:0 (Lipid numbers) | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.005.798 |
| EC Number | 206-376-4 |
| KEGG | |
| PubChem CID |
|
| RTECS number | HD9100000 |
| UNII | |
| |
| |
| Properties | |
| C10H20O2 | |
| Molar mass | 172.27 g·mol−1 |
| Appearance | White crystals |
| Odor | Strong rancid and unpleasant[2] |
| Density | 0.893 g/cm3 (25 °C)[3] 0.8884 g/cm3 (35.05 °C) 0.8773 g/cm3 (50.17 °C)[4] |
| Melting point | 31.6 °C (88.9 °F; 304.8 K)[5] |
| Boiling point | 268.7 °C (515.7 °F; 541.8 K) [6] |
| 0.015 g/100 mL (20 °C)[6] | |
| Solubility | Soluble in alcohol, ether, CHCl3, C6H6, CS2, acetone[2] |
| log P | 4.09[6] |
| Vapor pressure | 4.88·10−5 kPa (25 °C)[2] 0.1 kPa (108 °C)[6] 2.03 kPa (160 °C)[1][3] |
| Acidity (pKa) | 4.9[2] |
| Thermal conductivity | 0.372 W/m·K (solid) 0.141 W/m·K (liquid)[4] |
| Refractive index (nD) |
1.4288 (40 °C)[2] |
| Viscosity | 4.327 cP (50 °C)[6] 2.88 cP (70 °C)[4] |
| Structure | |
| Monoclinic (−3.15 °C)[7] | |
| P21/c[7] | |
| α = 90°, β = 91.28°, γ = 90° | |
| Thermochemistry | |
| 475.59 J/mol·K[1] | |
| Std enthalpy of formation (ΔfH |
−713.7 kJ/mol[6] |
| Std enthalpy of combustion (ΔcH |
6079.3 kJ/mol[1] |
| Hazards | |
| Main hazards | Medium toxicity |
| Safety data sheet | External MSDS |
| GHS pictograms |  [3] [3] |
| GHS signal word | Warning |
| H315, H319, H335[3] | |
| P261, P305+351+338[3] | |
| Ingestion hazard | May be toxic |
| Inhalation hazard | May cause irritation |
| Skin hazard | May be toxic on contact |
| NFPA 704 | |
| Flash point | 110 °C (230 °F; 383 K) [3] |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
10 g/kg (rats, oral)[8] |
| Related compounds | |
| Related fatty acids |
Caprylic acid Lauric acid |
| Related compounds |
Decanol Decanal |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Decanoic acid (capric acid) is a saturated fatty acid. Its formula is CH3(CH2)8COOH. Salts and esters of decanoic acid are called decanoates or "caprates". The term capric acid is derived from the Latin "caper / capra" (goat) because the sweaty, unpleasant smell of the compound is reminiscent of goats.[9]
Occurrence
Capric acid occurs naturally in coconut oil (about 10%) and palm kernel oil (about 4%), otherwise it is uncommon in typical seed oils.[10] It is found in the milk of various mammals and to a lesser extent in other animal fats.[5]
Two other acids are named after goats: caproic (a C6 fatty acid) and caprylic (a C8 fatty acid). Along with decanoic acid, these total 15% in goat milk fat.
Production
Decanoic acid can be prepared from oxidation of primary alcohol decanol by using chromium trioxide (CrO3) oxidant under acidic conditions.[11]
Neutralization of decanoic acid or saponification of its esters, typically triglycerides, with sodium hydroxide will give sodium decanoate. This salt (CH3(CH2)8COO−Na+) is a component of some types of soap.
Uses
Decanoic acid is used in the manufacture of esters for artificial fruit flavors and perfumes. It is also used as an intermediate in chemical syntheses. It is used in organic synthesis and industrially in the manufacture of perfumes, lubricants, greases, rubber, dyes, plastics, food additives and pharmaceuticals.[8]
Pharmaceuticals
Decanoate ester prodrugs of various pharmaceuticals are available. Since decanoic acid is a fatty acid, forming a salt or ester with a drug will increase its lipophilicity and its affinity for fatty tissue. Since distribution of a drug from fatty tissue is usually slow, one may develop a long-acting injectable form of a drug (called a Depot injection) by using its decanoate form. Some examples of drugs available as a decanoate ester include nandrolone, fluphenazine, bromperidol, and haloperidol.
Effects
Decanoic acid acts as a non-competitive AMPA receptor antagonist at therapeutically relevant concentrations, in a voltage- and subunit-dependent manner, and this is sufficient to explain its antiseizure effects.[12] This direct inhibition of excitatory neurotransmission by decanoic acid in the brain contributes to the anticonvulsant effect of the MCT ketogenic diet.[12] Decanoic acid and the AMPAr antagonist drug perampanel act at separate sites on the AMPA receptor, and so it is possible that they have a cooperative effect at the AMPA receptor, suggesting that perampanel and the ketogenic diet could be synergistic.[12]
Decanoic acid may be responsible for the mitochondrial proliferation associated with the ketogenic diet, and that this may occur via PPARγ receptor agonism and its target genes involved in mitochondrial biogenesis.[13][14] Complex I activity of the electron transport chain is substantially elevated by decanoic acid treatment.[13]
It should however be noted that orally ingested medium chain fatty acids would be very rapidly degraded by first-pass metabolism by being taken up in the liver via the portal vein, and are quickly metabolized via coenzyme A intermediates through β-oxidation and the citric acid cycle to produce carbon dioxide, acetate and ketone bodies.[15] It is unclear whether the ketones β-hydroxybutryate and acetone have direct antiseizure activity.[12][16][17][18]
See also
- Undecanoic acid
- Medium-chain triglyceride
- Nonanoic acid, a medium-chain fatty acid, also with antiseizure activity
References
- 1 2 3 4 n-Decanoic acid in Linstrom, P. J.; Mallard, W. G. (eds.) NIST Chemistry WebBook, NIST Standard Reference Database Number 69. National Institute of Standards and Technology, Gaithersburg MD. http://webbook.nist.gov (retrieved 2014-06-15)
- 1 2 3 4 5 CID 2969 from PubChem
- 1 2 3 4 5 6 Sigma-Aldrich Co., Decanoic acid. Retrieved on 2014-06-15.
- 1 2 3 Mezaki, Reiji; Mochizuki, Masafumi; Ogawa, Kohei (2000). Engineering Data on Mixing (1st ed.). Elsevier Science B.V. p. 278. ISBN 0-444-82802-8.
- 1 2 "Lexicon of lipid nutrition (IUPAC Technical Report)". Pure and Applied Chemistry. 73 (4): 685–744. 2001. doi:10.1351/pac200173040685.
- 1 2 3 4 5 6 Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- 1 2 3 Bond, Andrew D. (2003). "On the crystal structures and melting point alternation of the n-alkyl carboxylic acids" (PDF). http://www.rsc.org. Royal Society of Chemistry. Retrieved 2014-06-15. External link in
|website=(help) - 1 2 3 "CAPRIC ACID". http://www.chemicalland21.com. AroKor Holdings Inc. Retrieved 2014-06-15. External link in
|website=(help) - ↑ "capri-, capr- +". Retrieved 2012-09-28.
- ↑ David J. Anneken, Sabine Both, Ralf Christoph, Georg Fieg, Udo Steinberner, Alfred Westfechtel "Fatty Acids" in Ullmann's Encyclopedia of Industrial Chemistry, 2006, Wiley-VCH, Weinheim. doi:10.1002/14356007.a10_245.pub2
- ↑ John McMurry (2008). Organic Chemistry 7th edition. Thompson - Brooks/Cole. Page 624
- 1 2 3 4 Chang P, Augustin K, Boddum K, Williams S, Sun M, Terschak JA, Hardege JD, Chen PE, Walker MC, Williams RS (2016). "Seizure control by decanoic acid through direct AMPA receptor inhibition". Brain (journal). 139 (Pt 2): 431–443. PMC 4805082
 . PMID 26608744. doi:10.1093/brain/awv325.
. PMID 26608744. doi:10.1093/brain/awv325. - 1 2 Hughes SD, Kanabus M, Anderson G, Hargreaves IP, Rutherford T, O'Donnell M, Cross JH, Rahman S, Eaton S, Heales SJ (2014). "The ketogenic diet component decanoic acid increases mitochondrial citrate synthase and complex I activity in neuronal cells". Journal of Neurochemistry. 129 (3): 426–33. PMID 24383952. doi:10.1111/jnc.12646. Retrieved 2015-11-26.
- ↑ Malapaka RR, Khoo S, Zhang J, Choi JH, Zhou XE, Xu Y, Gong Y, Li J, Yong EL, Chalmers MJ, Chang L, Resau JH, Griffin PR, Chen YE, Xu HE (2012). "Identification and mechanism of 10-carbon fatty acid as modulating ligand of peroxisome proliferator-activated receptors". Journal of Biological Chemistry. 287 (1): 183–195. PMC 3249069
 . PMID 22039047. doi:10.1074/jbc.M111.294785.
. PMID 22039047. doi:10.1074/jbc.M111.294785. - ↑ Chang P, Terbach N, Plant N, Chen PE, Walker MC, Williams RS (2013). "Seizure control by ketogenic diet-associated medium chain fatty acids". Neuropharmacology. 69: 105–14. PMC 3625124
 . PMID 23177536. doi:10.1016/j.neuropharm.2012.11.004.
. PMID 23177536. doi:10.1016/j.neuropharm.2012.11.004. - ↑ Viggiano A, Pilla R, Arnold P, Monda M, D'Agostino D, Coppola G. "Anticonvulsant properties of an oral ketone ester in a pentylenetetrazole-model of seizure.". Brain Res. 1618: 50–4. PMID 26026798. doi:10.1016/j.brainres.2015.05.023.
- ↑ Rho JM, Anderson GD, Donevan SD, White HS. "Acetoacetate, acetone, and dibenzylamine (a contaminant in l-(+)-beta-hydroxybutyrate) exhibit direct anticonvulsant actions in vivo.". Epilepsia. 43: 358–61. PMID 11952765. doi:10.1046/j.1528-1157.2002.47901.x.
- ↑ Ma W, Berg J, Yellen G. "Ketogenic diet metabolites reduce firing in central neurons by opening K(ATP) channels.". J Neurosci. 27: 3618–25. PMID 17409226. doi:10.1523/JNEUROSCI.0132-07.2007.
