Instant coffee



Instant coffee, also called soluble coffee, coffee crystals, and coffee powder, is a beverage derived from brewed coffee beans that enables people to quickly prepare hot coffee by adding hot water to the powder or crystals and stirring. Instant coffee is commercially prepared by either freeze-drying or spray drying, after which it can be rehydrated. Instant coffee in a concentrated liquid form is also manufactured.[1]
Advantages of instant coffee include speed of preparation (instant coffee dissolves quickly in hot water), lower shipping weight and volume than beans or ground coffee (to prepare the same amount of beverage), and long shelf life—though instant coffee can spoil if not kept dry. Instant coffee also reduces cleanup since there are no coffee grounds, and at least one study has found that it has a lower environmental footprint than other preparation methods.[2]
History
Instant or soluble coffee was invented and patented in 1881, by Alphonse Allais, France, under patent number 141520.[3] In 1890, David Strang of Invercargill, New Zealand, under patent number 3518[4] sold under the trading name Strang's Coffee[5] citing the patented "Dry Hot-Air" process. The invention was previously attributed to Satori Kato, a Japanese scientist working in Chicago in 1901. Kato introduced the powdered substance in Buffalo, New York, at the Pan-American Exposition.[6] George Constant Louis Washington developed his own instant coffee process shortly thereafter, and first marketed it commercially (1910). The Nescafé brand, which introduced a more advanced coffee refining process, was launched in 1938.
High-vacuum freeze-dried coffee was developed shortly after World War II, as an indirect result of wartime research into other areas. The National Research Corporation (NRC) was formed in Massachusetts as a process-development company employing high-vacuum technology. It developed high-vacuum processes to produce penicillin, blood plasma, and streptomycin for US military use. As the war ended, NRC looked to adapt its processes for peacetime uses. It formed Florida Foods Corporation to produce concentrated orange juice powder, and originally sold its product to the United States Army. That company later changed its name to Minute Maid.
These days examples of popular instant coffee brands are Nescafé, International Roast, Extra, Folgers, Maxwell House, Robert Timms, and Starbucks VIA.
Use
Close to 50% of the world's green coffee is used to produce instant coffee.[7]
As food

Instant coffee is available in powder or granulated form contained in glass jars, sachets, or tins. The user controls the strength of the resulting product, by adding less or more powder to the water, ranging from thin "coffee water" to very strong and almost syrupy coffee.
Instant coffee is also convenient for preparing iced coffee like the Greek frappé.
In some countries, such as Portugal, Spain, and India, instant coffee is commonly mixed with hot milk instead of boiling water. In other countries, such as South Korea, instant coffee commonly comes pre-mixed with non-dairy creamer and sugar and is called "coffee mix".[8] Said to have been popularised in the UK by GIs during World War II, instant coffee still accounts for over 75 percent of coffee bought to drink in British homes, as opposed to well under 10 percent in the U.S. and France and one percent in Italy.[9]
Non-food use
Instant coffee is one of the ingredients in "Caffenol-C",[10] a home-made, non-toxic black-and-white photographic developer. The other ingredients in the basic formula are ascorbic acid and anhydrous sodium carbonate; some recipes also include potassium bromide as a fog-reducing agent. The active ingredient appears to be caffeic acid. Initial experiments on Caffenol were performed in 1995 at the Rochester Institute of Technology;[11] addition of ascorbic acid began around 2000, yielding the improved Caffenol-C, which is less likely to stain negatives than the original formulation. Experiments have shown that cheaper, less desirable brands of coffee work better for this application than more expensive brands.[12]
Production
As with regular coffee, the green coffee bean itself is first roasted to bring out flavour and aroma. Rotating cylinders containing the green beans and hot combustion gases are used in most roasting plants. When the bean temperature reaches 165 °C (329 °F) the roasting begins. It takes about 8–15 minutes to complete roasting. After cooling, the beans are then ground finely. Grinding reduces the beans to 0.5–1.1-millimetre (0.020–0.043 in) pieces. Until here, the process is in general the same as for other types of coffee.[13]
Extraction
To produce instant coffee, the soluble and volatile contents of the beans, which provide the coffee aroma and flavor, have to be extracted. This is done using water. Pressurized liquid water heated to around 175 °C (347 °F) is used for this process. The coffee concentration in the liquid is then increased by either evaporation or by freeze concentration.[13][14]
Freeze drying
The basic principle of freeze drying is the removal of water by sublimation.
Since the mass production of instant coffee began in post-WWII America, freeze-drying has grown in popularity to become a common method. Although it is more expensive, it generally results in a higher-quality product.
- The coffee extract is rapidly frozen and is broken into small granules. (Slower freezing would lead to larger ice crystals and a porous product; it can also affect the colour of the coffee granules).
- The granules are sifted and sorted on size.[13]
- Frozen coffee granules are placed in the drying chamber, often on metal trays.
- A vacuum is created within the chamber. The strength of the vacuum is critical in the speed of the drying and therefore the quality of the product. Care must be taken to produce a vacuum of suitable strength.
- The drying chamber is warmed, most commonly by radiation, but conduction is used in some plants and convection has been proposed in some small pilot plants. A possible problem with convection is uneven drying rates within the chamber, which would give an inferior product.
- Condensation—the previously frozen water in the coffee granules expands to ten times its previous volume. The removal of this water vapor from the chamber is vitally important, making the condenser the most critical and expensive component in a freeze-drying plant.
- The freeze-dried granules are removed from the chamber and packaged.
Spray drying
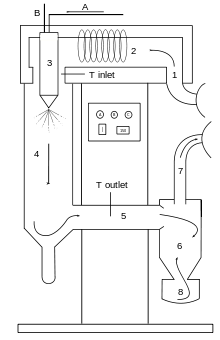
A=Solution or suspension to be dried in, B=Atomization gas in, 1= Drying gas in, 2=Heating of drying gas, 3=Spraying of solution or suspension, 4=Drying chamber, 5=Part between drying chamber and cyclone, 6=Cyclone, 7=Drying gas is taken away, 8=Collection vessel of product, arrows mean that this is co-current lab-spraydryer
Spray drying is preferred to freeze drying in some cases because it allows larger scale economic production, shorter drying times, and because it produces fine, rounded particles.
The process produces spherical particles about 300 micrometres (0.012 in) in size with a density of 0.22 g/cm³.[15] To achieve this, nozzle atomization is used. Various ways of nozzle atomization can be used each having its own advantages and disadvantages. High speed rotating wheels operating at speeds of about 20,000 rpm are able to process up to 60,000 pounds (27 tonnes) of solution per hour.[16] The use of spray wheels requires that the drying towers have a wide radius to avoid the atomized droplets collecting onto the drying chamber walls.
- Completed in 5–30 seconds (dependent on factors such as heat, size of particle, and diameter of chamber).
- Moisture content change: IN = 75-85% OUT = 3-3.5%
- Air temperature: IN = 270 °C (518 °F) OUT = 110 °C (230 °F)
One drawback with spray drying is that the particles it produces are too fine to be used effectively by the consumer; they must first be either steam-fused in towers similar to spray dryers or by belt agglomeration to produce particles of suitable size.
Decaffeination
In commercial processes, the decaffeination of instant coffee almost always happens before the critical roasting process which will determine the coffee's flavour and aroma processes.
Byproducts
The main byproduct of the instant coffee production process is spent coffee powder. This powder can be used as biomass, for example to produce heat used in the manufacturing process.[17] Roughly 2 times the mass in spent coffee powder is generated for each quantity of soluble coffee.[18]
Composition
.jpg)
The caffeine content of instant coffee is generally less than that of brewed coffee. One study comparing various home-prepared samples came to the result that regular instant coffee (not decaffeinated) has a median caffeine content of 66 mg per cup (range 29–117 mg per cup), with a median cup size of 225 ml (range 170-285 ml) and a caffeine concentration of 328 µg/ml (range 102-559 µg/ml).[19] In comparison, drip or filter coffee was estimated to have a median caffeine content of 112 mg, with a median concentration of 621 µg/ml for the same cup size.[19]
Regarding antioxidants, the polyphenol content of a 180 ml cup of instant coffee has been estimated to be approximately 320 mg, compared to approximately 400 mg in a cup of brewed coffee of the same size.[20]
Health hazards
Malabsorption
Instant coffee decreases intestinal iron absorption more than drip coffee. One study estimated that, when a cup of instant coffee was ingested with a meal composed of semipurified ingredients, intestinal absorption was reduced from 5.88% to 0.97%, compared to an absorption of 1.64% with drip coffee.[21] It was also estimated that, when the strength of the instant coffee was doubled, intestinal iron absorption fell to 0.53%.[21] Apparently, however, there is no decrease in iron absorption when instant coffee is consumed 1 hour before a meal, but the same degree of inhibition as with simultaneous ingestion occurs when instant coffee is taken 1 hour after a meal.[21]
Carcinogenicity
Instant coffee has been associated with an increased risk of bladder cancer in women when compared to regular coffee, whereas for men both instant and regular coffee have been associated with an increased bladder cancer risk.[22] However, current review research suggests that there is no dose-response relationship between coffee drinking and bladder cancer, and that previous studies may have been confounded by unidentified risks of bladder cancer.[23]
Per an FDA survey, brewed instant coffee has acrylamide levels of 3-7 ppb which is less than brewed regular coffee, i.e. 6-13 ppb.[24] If comparing coffee as a solid, instant coffee crystals have acrylamide levels of 172-539 ng/g whereas unbrewed regular coffee grounds have 45-374 ng/g.[25]
Regulation
In the EU, regulations include the following details:
- Species of coffee bean
- Geographical origin
- Processing detail
- Year of crop
- Solvents used in decaffeination
- Caffeine level
Various institutions govern the coffee industry and help to achieve standardisation and also release information to the public.
- International Coffee Organisation (London)
- Codex Alimentarius Commission of the UN (Rome)
- National Coffee Association (New York)
See also
References
- ↑ Cafe Industry (2011-08-15). "TORQ Natural Instant Coffee". Cafe Culture. Retrieved 2012-10-22.
- ↑ Humbert, Sebastien; Loerincik, Yves; Rossi, Vincent; Margni, Manuele; Jolliet, Olivier (2009). "Life cycle assessment of spray dried soluble coffee and comparison with alternatives (drip filter and capsule espresso)". Journal of Cleaner Production. 17 (15): 1351–1358. ISSN 0959-6526. doi:10.1016/j.jclepro.2009.04.011.
- ↑ Dominique Bougerie, « Honfleur et les honfleurais. Cinq siècles d'histoires », Ed. Marie, 2002, p. 138
- ↑ "First Annual Report". Patents, Designs and Trade-marks. New Zealand. 1890. p. 9.
- ↑ Papers Past — Press — 7 September 1893 — Page 3 Advertisements Column 2
- ↑ Carlisle, Rodney (2004). Scientific American Inventions and Discoveries, p.355. John Wiley & Songs, Inc., New Jersey. ISBN 0-471-24410-4.
- ↑ Ramalakshmi, K.; Rao, L. Jagan Mohan; Takano-Ishikawa, Yuko; Goto, Masao (2009). "Bioactivities of low-grade green coffee and spent coffee in different in vitro model systems". Food Chemistry. 115 (1): 79–85. ISSN 0308-8146. doi:10.1016/j.foodchem.2008.11.063.
- ↑ "Koreans Addicted to Instant Coffee". Koreatimes.co.kr. 2009-04-22. Retrieved 2013-11-17.
- ↑ Magazine Monitor. "Why do Britons drink so much instant coffee?". BBC News Magazine. Retrieved 5 April 2014.
- ↑ Comparison of different Caffenol formulas
- ↑ A Use for that Last Cup of Coffee: Film and Paper Development Darkroom and Creative Camera Techniques, September/October 1995
- ↑ One recipe for Caffenol-C
- 1 2 3 Mussatto, Solange I.; Machado, Ercília M. S.; Martins, Silvia; Teixeira, José A. (2011). "Production, Composition, and Application of Coffee and Its Industrial Residues". Food and Bioprocess Technology. 4 (5): 661–672. ISSN 1935-5130. doi:10.1007/s11947-011-0565-z.
- ↑ How It Works - Instant Coffee (video). Retrieved 4 March 2016.
- ↑ Masters, K (1991). Spray Drying Handbook (5th ed.). Longman Scientific & Technical. ISBN 0-582-06266-7.
- ↑ John J. McKetta, ed. (1995). Encyclopedia of Chemical Processing and Design. Marcel Dekker Inc. ISBN 0-8247-2604-9.
- ↑ "Instant Coffee". Madehow.
- ↑ Pfluger, R. A. (1975). Soluble coffee processing. In C. L. Mantell (Ed.), Solid wastes: origin, collection, processing, and disposal. New York: Wiley.
- 1 2 Gilbert, R.; Marshman, J.; Schwieder, M.; Berg, R. (1976). "Caffeine content of beverages as consumed". Canadian Medical Association journal. 114 (3): 205–208. PMC 1956955
 . PMID 1032351.
. PMID 1032351. - ↑ Bonita, J.; Mandarano, M.; Shuta, D.; Vinson, J. (2007). "Coffee and cardiovascular disease: In vitro, cellular, animal, and human studies". Pharmacological Research. 55 (3): 187–198. PMID 17368041. doi:10.1016/j.phrs.2007.01.006.
- 1 2 3 Morck, T.; Lynch, S.; Cook, J. (1983). "Inhibition of food iron absorption by coffee". The American Journal of Clinical Nutrition. 37 (3): 416–420. PMID 6402915.
- ↑ Howe, G.; Burch, J.; Miller, A.; Cook, G.; Esteve, J.; Morrison, B.; Gordon, P.; Chambers, L.; Fodor, G.; Winsor, G. M. (1980). "Tobacco use, occupation, coffee, various nutrients, and bladder cancer". Journal of the National Cancer Institute. 64 (4): 701–713. PMID 6928984.
- ↑ Pelucchi, C; La Vecchia, C (2012-05-24). "Alcohol, coffee, and bladder cancer risk: a review of epidemiological studies". Eur. J. Cancer Prev. 18: 62–8. PMID 19077567. doi:10.1097/CEJ.0b013e32830c8d44.
- ↑ "Survey Data on Acrylamide in Food: Individual Food Products". Table 3: Acrylamide values in food product samples (data collected between February 8, 2003 and October 1, 2003). Retrieved 2015-06-15.
- ↑ Andrzejewski D, Roach JA, Gay ML, Musser SM (2004). "Analysis of coffee for the presence of acrylamide by LC-MS/MS". Journal of Agricultural and Food Chemistry. 52 (7): 1996–2002. PMID 15053542. doi:10.1021/jf0349634. Retrieved 2015-06-15.
Bibliography
- Romualdo Verzosa Jr., ed. (1993). Encyclopedia of Chemical Technology, volume 6 (4th ed.). John Wiley & Sons. ISBN 0-471-52674-6.
- Masters, K (1991). Spray Drying Handbook (5th ed.). Longman Scientific & Technical. ISBN 0-582-06266-7.
- John J. McKetta, ed. (1995). Encyclopedia of Chemical Processing and Design. Marcel Dekker Inc. ISBN 0-8247-2604-9.
External links
| Wikimedia Commons has media related to Instant coffee. |

