Decitabine
 | |
| Clinical data | |
|---|---|
| Trade names | Dacogen |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608009 |
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | <1% |
| Biological half-life | 30 minutes |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.017.355 |
| Chemical and physical data | |
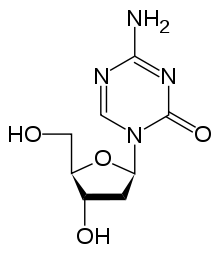
| Formula | C8H12N4O4 |
| Molar mass | 228.206 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Decitabine (trade name Dacogen), or 5-aza-2'-deoxycytidine, acts as an Nucleic Acid Synthesis Inhibitor.[1] It is a drug for the treatment of myelodysplastic syndromes, a class of conditions where certain blood cells are dysfunctional, and for acute myeloid leukemia (AML).[2] Chemically, it is a cytidine analog.
Mechanism
Decitabine is a hypomethylating agent.[3][4] It hypomethylates DNA by inhibiting DNA methyltransferase.
It functions in a similar manner to azacitidine, although decitabine can only be incorporated into DNA strands while azacitidine can be incorporated into both DNA and RNA chains.
Clinical uses
Decitabine is indicated for the treatment of myelodysplastic syndromes (MDS) including previously treated and untreated, de novo and secondary MDS of all French-American-British subtypes (refractory anemia, refractory anemia with ringed sideroblasts, refractory anemia with excess blasts, refractory anemia with excess blasts in transformation, and chronic myelomonocytic leukemia) and Intermediate-1, Intermediate-2, and High-Risk International Prognostic Scoring System groups. In patients with renal insufficiency, Batty and colleagues reported the first case series on the feasibility of therapy with hypomethylating agents in patients with renal insufficiency.[5]
It also has EU approval for acute myeloid leukemia (AML).[2]
Research - atherosclerosis
A number of investigators have shown a relationship between atherosclerosis and disturbed blood flow. This upregulates DNA methyltransferase expression, which leads to genome-wide DNA methylation alterations and global gene expression changes. These studies have revealed several mechanosensitive genes, such as HoxA5, Klf3, and Klf4, whose promoters were hypermethylated by disturbed blood flow, but rescued by DNA methyltransferases inhibitors such as 5-aza-2'-deoxycytidine. It has been found that use of this DNA methyltranferase inhibitor prevents atherosclerosis lesion formation and reduces the production of inflammatory cytokines by macrophages.[6]
References
- ↑ "Decitabine". National Center for Biotechnology Information. Retrieved September 24, 2016.
- 1 2 "EC Approves Marketing Authorization Of DACOGEN For Acute Myeloid Leukemia". 2012-09-28. Retrieved 28 September 2012.
- ↑ Kantarjian H, Issa JP, Rosenfeld CS, et al. (April 2006). "Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study". Cancer. 106 (8): 1794–1803. PMID 16532500. doi:10.1002/cncr.21792.
- ↑ Kantarjian HM, O'Brien S, Cortes J, et al. (August 2003). "Results of decitabine (5-aza-2'deoxycytidine) therapy in 130 patients with chronic myelogenous leukemia". Cancer. 98 (3): 522–528. PMID 12879469. doi:10.1002/cncr.11543.
- ↑ Ravandi, F.; Kantarjian, J. E.; Issa, S.; Jabbour, S.; Santos, G.; McCue, D.; Garcia-Manero, F. P. S.; Pierce, E.; O'Brien, J. P.; Cortés, J. E.; Ravandi, F. (2010). "Feasibility of Therapy with Hypomethylating Agents in Patients with Renal Insufficiency". Clinical Lymphoma, Myeloma & Leukemia. 10 (3): 205–210. PMC 3726276
 . PMID 20511166. doi:10.3816/CLML.2010.n.032.
. PMID 20511166. doi:10.3816/CLML.2010.n.032. - ↑ Dunn, J; Thabet, S; Jo, H (July 2015). "Flow-Dependent Epigenetic DNA Methylation in Endothelial Gene Expression and Atherosclerosis.". Arteriosclerosis, thrombosis, and vascular biology. 35 (7): 1562–9. PMC 4754957
 . PMID 25953647.
. PMID 25953647.
Further reading
- Moon C, Kim SH (June 2009). "Use of epigenetic modification to induce FOXP3 expression in naïve T cells". Transplant Proc. 41 (5): 1848–1854. PMID 19545742. doi:10.1016/j.transproceed.2009.02.101.