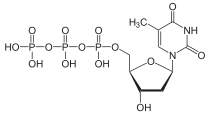
Thymidine triphosphate
 | |
 | |
| Names | |
|---|---|
| IUPAC name
[(2R,3S,5R)-3-hydroxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methyl (hydroxy-phosphonooxyphosphoryl) hydrogen phosphate | |
| Other names
dTTP, 2'-deoxythymidine triphosphate | |
| Identifiers | |
| |
| 3D model (JSmol) |
|
| ChemSpider |
|
| ECHA InfoCard | 100.006.064 |
| MeSH | thymidine+5'-triphosphate |
| PubChem CID |
|
| |
| Properties | |
| C10H17N2O14P3 | |
| Molar mass | 482.168 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Deoxythymidine triphosphate (dTTP) is one of the four nucleoside triphosphates that are used in the in vivo synthesis of DNA. Unlike the other deoxyribonucleoside triphosphates, thymidine triphosphate does not always contain the "deoxy" prefix in its name.[1] The corresponding ribonucleoside triphosphate is called uridine triphosphate.
It can be used by DNA ligase to create overlapping "sticky ends" so that protruding ends of opened microbial plasmids may be closed up.
References
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.