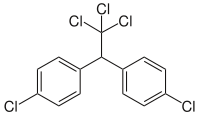
Dichlorodiphenyltrichloroethane
 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1,1'-(2,2,2-Trichloroethane-1,1-diyl)bis(4-chlorobenzene) | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.000.023 |
| KEGG | |
| PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C14H9Cl5 | |
| Molar mass | 354.48 g·mol−1 |
| Density | 0.99 g/cm3 |
| Melting point | 108.5 °C (227.3 °F; 381.6 K) |
| Boiling point | 260 °C (500 °F; 533 K) (decomposes) |
| 25 μg/L (25 °C)[1] | |
| Hazards | |
| Main hazards | Toxic, dangerous to the environment, likely carcinogenic |
| EU classification (DSD) (outdated) |
|
| R-phrases (outdated) | R25 R40 R48/25 R50/53 |
| S-phrases (outdated) | (S1/2) S22 S36/37 S45 S60 S61 |
| NFPA 704 | |
| Flash point | 72–77 °C; 162–171 °F; 345–350 K [2] |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
113–800 mg/kg (rat, oral)[1] 250 mg/kg (rabbit, oral) 135 mg/kg (mouse, oral) 150 mg/kg (guinea pig, oral)[3] |
| US health exposure limits (NIOSH):[4] | |
| PEL (Permissible) |
TWA 1 mg/m3 [skin] |
| REL (Recommended) |
Ca TWA 0.5 mg/m3 |
| IDLH (Immediate danger) |
500 mg/m3 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Dichlorodiphenyltrichloroethane (DDT) is a colorless, tasteless, and almost odorless crystalline organochlorine known for its insecticidal properties and environmental impacts. First synthesized in 1874, DDT's insecticidal action was discovered by the Swiss chemist Paul Hermann Müller in 1939. DDT was used in the second half of World War II to control malaria and typhus among civilians and troops. Müller was awarded the Nobel Prize in Physiology or Medicine "for his discovery of the high efficiency of DDT as a contact poison against several arthropods" in 1948.[5]
By October 1945, DDT was available for public sale in the United States. Although it was promoted by government and industry for use as an agricultural and household pesticide, there were also concerns about its use from the beginning.[6] Opposition to DDT was focused by the 1962 publication of Rachel Carson's book Silent Spring. It cataloged environmental impacts that coincided with widespread use of DDT in agriculture in the United States, and it questioned the logic of broadcasting potentially dangerous chemicals into the environment with little prior investigation of their environment and health effects. The book claimed that DDT and other pesticides had been shown to cause cancer and that their agricultural use was a threat to wildlife, particularly birds. Its publication was a seminal event for the environmental movement and resulted in a large public outcry that eventually led, in 1972, to a ban on DDT's agricultural use in the United States.[7] A worldwide ban on agricultural use was formalized under the Stockholm Convention on Persistent Organic Pollutants, but its limited and still-controversial use in disease vector control continues,[8][9] because of its effectiveness in reducing malarial infections, balanced by environmental and other health concerns.
Along with the passage of the Endangered Species Act, the United States ban on DDT is a major factor in the comeback of the bald eagle (the national bird of the United States) and the peregrine falcon from near-extinction in the contiguous United States.[10][11]
Properties and chemistry
DDT is similar in structure to the insecticide methoxychlor and the acaricide dicofol. It is highly hydrophobic and nearly insoluble in water but has good solubility in most organic solvents, fats and oils. DDT does not occur naturally and is synthesised by a Friedel–Crafts hydroxyalkylation reaction between chloral (CCl
3CHO) and chlorobenzene (C
6H
5Cl), in the presence of an acidic catalyst. DDT has been marketed under trade names including Anofex, Cezarex, Chlorophenothane, Clofenotane, Dicophane, Dinocide, Gesarol, Guesapon, Guesarol, Gyron, Ixodex, Neocid, Neocidol and Zerdane.[12]
Isomers and related compounds
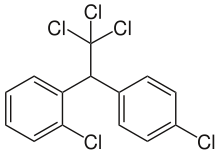
Commercial DDT is a mixture of several closely–related compounds. The major component (77%) is the p,p' isomer (pictured above). The o,p' isomer (pictured to the right) is also present in significant amounts (15%). Dichlorodiphenyldichloroethylene (DDE) and dichlorodiphenyldichloroethane (DDD) make up the balance. DDE and DDD are the major metabolites and environmental breakdown products.[12]
Production and use
DDT has been formulated in multiple forms, including solutions in xylene or petroleum distillates, emulsifiable concentrates, water-wettable powders, granules, aerosols, smoke candles and charges for vaporizers and lotions.[13]
From 1950 to 1980, DDT was extensively used in agriculture – more than 40,000 tonnes each year worldwide[14] – and it has been estimated that a total of 1.8 million tonnes have been produced globally since the 1940s.[1] In the United States, it was manufactured by some 15 companies, including Monsanto,[15] Ciba,[16] Montrose Chemical Company, Pennwalt[17] and Velsicol Chemical Corporation.[18] Production peaked in 1963 at 82,000 tonnes per year.[12] More than 600,000 tonnes (1.35 billion pounds) were applied in the US before the 1972 ban. Usage peaked in 1959 at about 36,000 tonnes.[19]
In 2009, 3,314 tonnes were produced for malaria control and visceral leishmaniasis. India is the only country still manufacturing DDT and is the largest consumer.[20] China ceased production in 2007.[21]
Mechanism of insecticide action
In insects it opens sodium ion channels in neurons, causing them to fire spontaneously, which leads to spasms and eventual death.[22] Insects with certain mutations in their sodium channel gene are resistant to DDT and similar insecticides. DDT resistance is also conferred by up-regulation of genes expressing cytochrome P450 in some insect species,[23] as greater quantities of some enzymes of this group accelerate the toxin's metabolism into inactive metabolites. (The same enzyme family is up-regulated in mammals too, e.g., in response to ethanol consumption.) Genomic studies in the model genetic organism Drosophila melanogaster revealed that high level DDT resistance is polygenic, involving multiple resistance mechanisms.[24]
History

|
| |
|
|
DDT was first synthesized in 1874 by Othmar Zeidler under the supervision of Adolf von Baeyer.[25][26] It was further described in 1929 in a dissertation by W. Bausch and in two subsequent publications in 1930.[27][28] The insecticide properties of "multiple chlorinated aliphatic or fat-aromatic alcohols with at least one trichloromethane group" were described in a patent in 1934 by Wolfgang von Leuthold.[29] DDT's insecticidal properties were not, however, discovered until 1939 by the Swiss scientist Paul Hermann Müller, who was awarded the 1948 Nobel Prize in Physiology and Medicine for his efforts.[5]
Use in the 1940s and 1950s
.jpg)
DDT is the best-known of several chlorine-containing pesticides used in the 1940s and 1950s. With pyrethrum in short supply, DDT was used extensively during World War II by the Allies to control the insect vectors of typhus – nearly eliminating the disease in many parts of Europe. In the South Pacific, it was sprayed aerially for malaria and dengue fever control with spectacular effects. While DDT's chemical and insecticidal properties were important factors in these victories, advances in application equipment coupled with competent organization and sufficient manpower were also crucial to the success of these programs.[30]
In 1945, DDT was made available to farmers as an agricultural insecticide[12] and played a role in the final elimination of malaria in Europe and North America.[8][31][32]
In 1955, the World Health Organization commenced a program to eradicate malaria in countries with low to moderate transmission rates worldwide, relying largely on DDT for mosquito control and rapid diagnosis and treatment to reduce transmission.[33] The program eliminated the disease in "Taiwan, much of the Caribbean, the Balkans, parts of northern Africa, the northern region of Australia, and a large swath of the South Pacific"[34] and dramatically reduced mortality in Sri Lanka and India.[35]
However, failure to sustain the program, increasing mosquito tolerance to DDT, and increasing parasite tolerance led to a resurgence. In many areas early successes partially or completely reversed, and in some cases rates of transmission increased.[36] The program succeeded in eliminating malaria only in areas with "high socio-economic status, well-organized healthcare systems, and relatively less intensive or seasonal malaria transmission".[37]
DDT was less effective in tropical regions due to the continuous life cycle of mosquitoes and poor infrastructure. It was not applied at all in sub-Saharan Africa due to these perceived difficulties. Mortality rates in that area never declined to the same dramatic extent, and now constitute the bulk of malarial deaths worldwide, especially following the disease's resurgence as a result of resistance to drug treatments and the spread of the deadly malarial variant caused by Plasmodium falciparum.
Eradication was abandoned in 1969 and attention instead focused on controlling and treating the disease. Spraying programs (especially using DDT) were curtailed due to concerns over safety and environmental effects, as well as problems in administrative, managerial and financial implementation.[36] Efforts shifted from spraying to the use of bednets impregnated with insecticides and other interventions.[37][38]
United States ban
By October 1945, DDT was available for public sale in the United States, used both as an agricultural pesticide and as a household insecticide.[6] Although its use was promoted by government and the agricultural industry, US scientists such as FDA pharmacologist Herbert O. Calvery expressed concern over possible hazards associated with DDT as early as 1944.[39][19][6] As its production and use increased, public response was mixed. At the same time that DDT was hailed as part of the "world of tomorrow," concerns were expressed about its potential to kill harmless and beneficial insects (particularly pollinators), birds, fish, and eventually humans. The issue of toxicity was complicated, partly because DDT's effects varied from species to species, and partly because consecutive exposures could accumulate, causing damage comparable to large doses. A number of states attempted to regulate DDT.[6][12] In the 1950s the federal government began tightening regulations governing its use.[19] These events received little attention. Women like Dorothy Colson and Mamie Ella Plyler of Claxton, Georgia gathered evidence about DDT's effects and wrote to the Georgia Department of Public Health, the National Health Council in New York City, and other organizations.[40]
In 1957 the New York Times reported an unsuccessful struggle to restrict DDT use in Nassau County, New York, and the issue came to the attention of the popular naturalist-author Rachel Carson. William Shawn, editor of The New Yorker, urged her to write a piece on the subject, which developed into her 1962 book Silent Spring. The book argued that pesticides, including DDT, were poisoning both wildlife and the environment and were endangering human health.[7] Silent Spring was a best seller, and public reaction to it launched the modern environmental movement in the United States. The year after it appeared, President John F. Kennedy ordered his Science Advisory Committee to investigate Carson's claims. The committee's report "add[ed] up to a fairly thorough-going vindication of Rachel Carson’s Silent Spring thesis," in the words of the journal Science,[41] and recommended a phaseout of "persistent toxic pesticides".[42]
DDT became a prime target of the growing anti-chemical and anti-pesticide movements, and in 1967 a group of scientists and lawyers founded the Environmental Defense Fund (EDF) with the specific goal of enacting a ban on DDT. Victor Yannacone, Charles Wurster, Art Cooley and others in the group had all witnessed bird kills or declines in bird populations and suspected that DDT was the cause. In their campaign against the chemical, EDF petitioned the government for a ban and filed lawsuits.[43] Around this time, toxicologist David Peakall was measuring DDE levels in the eggs of peregrine falcons and California condors and finding that increased levels corresponded with thinner shells.
In response to an EDF suit, the U.S. District Court of Appeals in 1971 ordered the EPA to begin the de-registration procedure for DDT. After an initial six-month review process, William Ruckelshaus, the Agency's first Administrator rejected an immediate suspension of DDT's registration, citing studies from the EPA's internal staff stating that DDT was not an imminent danger.[19] However, these findings were criticized, as they were performed mostly by economic entomologists inherited from the United States Department of Agriculture, who many environmentalists felt were biased towards agribusiness and understated concerns about human health and wildlife. The decision thus created controversy.[30]
The EPA held seven months of hearings in 1971–1972, with scientists giving evidence for and against DDT. In the summer of 1972, Ruckelshaus announced the cancellation of most uses of DDT – exempting public health uses under some conditions.[19] Immediately after the announcement, both EDF and the DDT manufacturers filed suit against EPA. Industry sought to overturn the ban, while EDF wanted a comprehensive ban. The cases were consolidated, and in 1973 the United States Court of Appeals for the District of Columbia Circuit ruled that the EPA had acted properly in banning DDT.[19]
Some uses of DDT continued under the public health exemption. For example, in June 1979, the California Department of Health Services was permitted to use DDT to suppress flea vectors of bubonic plague.[44] DDT continued to be produced in the United States for foreign markets until 1985, when over 300 tons were exported.[1]
Restrictions on usage
In the 1970s and 1980s, agricultural use was banned in most developed countries, beginning with Hungary in 1968[45] followed by Norway and Sweden in 1970, West Germany and the US in 1972, but not in the United Kingdom until 1984. By 1991 total bans, including for disease control, were in place in at least 26 countries; for example Cuba in 1970, Singapore in 1984, Chile in 1985 and the Republic of Korea in 1986.[46]
The Stockholm Convention on Persistent Organic Pollutants, which took effect in 2004, put a global ban on several persistent organic pollutants, and restricted DDT use to vector control. The Convention was ratified by more than 170 countries. Recognizing that total elimination in many malaria-prone countries is currently unfeasible absent affordable/effective alternatives, the convention exempts public health use within World Health Organization (WHO) guidelines from the ban.[47] Resolution 60.18 of the World Health Assembly commits WHO to the Stockholm Convention's aim of reducing and ultimately eliminating DDT.[48] Malaria Foundation International states, "The outcome of the treaty is arguably better than the status quo going into the negotiations. For the first time, there is now an insecticide which is restricted to vector control only, meaning that the selection of resistant mosquitoes will be slower than before."[49]
Despite the worldwide ban, agricultural use continued in India,[50] North Korea, and possibly elsewhere as of 2008.[20]
Today, about 3,000 to 4,000 tons of DDT are produced each year for disease vector control.[21] DDT is applied to the inside walls of homes to kill or repel mosquitoes. This intervention, called indoor residual spraying (IRS), greatly reduces environmental damage. It also reduces the incidence of DDT resistance.[51] For comparison, treating 40 hectares (99 acres) of cotton during a typical U.S. growing season requires the same amount of chemical as roughly 1,700 homes.[52]
Environmental impact

DDT is a persistent organic pollutant that is readily adsorbed to soils and sediments, which can act both as sinks and as long-term sources of exposure affecting organisms.[13] Depending on conditions, its soil half-life can range from 22 days to 30 years. Routes of loss and degradation include runoff, volatilization, photolysis and aerobic and anaerobic biodegradation. Due to hydrophobic properties, in aquatic ecosystems DDT and its metabolites are absorbed by aquatic organisms and adsorbed on suspended particles, leaving little DDT dissolved in the water. Its breakdown products and metabolites, DDE and DDD, are also persistent and have similar chemical and physical properties.[1] DDT and its breakdown products are transported from warmer areas to the Arctic by the phenomenon of global distillation, where they then accumulate in the region's food web.[53]
Because of its lipophilic properties, DDT can bioaccumulate, especially in predatory birds.[54] DDT is toxic to a wide range of living organisms, including marine animals such as crayfish, daphnids, sea shrimp and many species of fish. DDT, DDE and DDD magnify through the food chain, with apex predators such as raptor birds concentrating more chemicals than other animals in the same environment. They are stored mainly in body fat. DDT and DDE are resistant to metabolism; in humans, their half-lives are 6 and up to 10 years, respectively. In the United States, these chemicals were detected in almost all human blood samples tested by the Centers for Disease Control in 2005, though their levels have sharply declined since most uses were banned.[55] Estimated dietary intake has declined,[55] although FDA food tests commonly detect it.[56]
Eggshell thinning
The chemical and its breakdown products DDE and DDD caused eggshell thinning and population declines in multiple North American and European bird of prey species.[1][57][10][58][59][60] DDE-related eggshell thinning is considered a major reason for the decline of the bald eagle,[10] brown pelican,[61] peregrine falcon and osprey.[1] However, birds vary in their sensitivity to these chemicals, with birds of prey, waterfowl and song birds being more susceptible than chickens and related species.[1][13] Even in 2010, California condors that feed on sea lions at Big Sur that in turn feed in the Palos Verdes Shelf area of the Montrose Chemical Superfund site exhibited continued thin-shell problems,[62][63] though DDT's role in the decline of the California Condor is disputed.[60][59]
The biological thinning mechanism is not entirely understood, but DDE appears to be more potent than DDT,[1] and strong evidence indicates that p,p'-DDE inhibits calcium ATPase in the membrane of the shell gland and reduces the transport of calcium carbonate from blood into the eggshell gland. This results in a dose-dependent thickness reduction.[1][64][65][58] Other evidence indicates that o,p'-DDT disrupts female reproductive tract development, later impairing eggshell quality.[66] Multiple mechanisms may be at work, or different mechanisms may operate in different species.[1]
Human health
DDT is an endocrine disruptor.[67][68] It is considered likely to be a human carcinogen although the majority of studies suggest it is not directly genotoxic.[69][70][71] DDE acts as a weak androgen receptor antagonist, but not as an estrogen.[72] p,p'-DDT, DDT's main component, has little or no androgenic or estrogenic activity.[73] The minor component o,p'-DDT has weak estrogenic activity.
Acute toxicity
DDT is classified as "moderately toxic" by the US National Toxicology Program (NTP)[74] and "moderately hazardous" by WHO, based on the rat oral LD50 of 113 mg/kg.[75] Indirect exposure is considered relatively non-toxic for humans.[76]
Chronic toxicity
Primarily through the tendency for DDT to buildup in areas of the body with high lipid content, chronic exposure can affect reproductive capabilities and the embryo or fetus.[76]
- A review article in The Lancet states, "research has shown that exposure to DDT at amounts that would be needed in malaria control might cause preterm birth and early weaning ... toxicological evidence shows endocrine-disrupting properties; human data also indicate possible disruption in semen quality, menstruation, gestational length, and duration of lactation."[38]
- Other studies document decreases in semen quality among men with high exposures (generally from IRS).[77]
- Studies are inconsistent on whether high blood DDT or DDE levels increase time to pregnancy.[55] In mothers with high DDE blood serum levels, daughters may have up to a 32% increase in the probability of conceiving, but increased DDT levels have been associated with a 16% decrease in one study.[78]
- Indirect exposure of mothers through workers directly in contact with DDT is associated with an increase in spontaneous abortions[76]
- Other studies found that DDT or DDE interfere with proper thyroid function in pregnancy and childhood.[55][79]
Carcinogenicity
In 2015, the International Agency for Research on Cancer classifies DDT as Group 2A "probably carcinogenic to humans".[80] Previous assessments by the U.S. National Toxicology Program classified it as "reasonably anticipated to be a carcinogen" and by the EPA classified DDT, DDE and DDD as class B2 "probable" carcinogens; these evaluations were based mainly on animal studies.[1][38]
A 2005 Lancet review stated that occupational DDT exposure was associated with increased pancreatic cancer risk in 2 case control studies, but another study showed no DDE dose-effect association. Results regarding a possible association with liver cancer and biliary tract cancer are conflicting: workers who did not have direct occupational DDT contact showed increased risk. White men had an increased risk, but not white women or black men. Results about an association with multiple myeloma, prostate and testicular cancer, endometrial cancer and colorectal cancer have been inconclusive or generally do not support an association.[38] A 2017 review of liver cancer studies concluded that "organochlorine pesticides, including DDT, may increase hepatocellular carcinoma risk."[81]
A 2009 review, whose co-authors included persons engaged in DDT-related litigation, reached broadly similar conclusions, with an equivocal association with testicular cancer. Case–control studies did not support an association with leukemia or lymphoma.[55]
Breast cancer
The question of whether DDT or DDE are risk factors in breast cancer has not been conclusively answered. Several meta analyses of observational studies have concluded that there is no overall relationship between DDT exposure and breast cancer risk.[82][83] The United States Institute of Medicine reviewed data on the association of breast cancer with DDT exposure in 2012 and concluded that a causative relationship could neither be proven nor disproven.[84]
A 2007 case control study[73] using archived blood samples found that breast cancer risk was increased 5-fold among women who were born prior to 1931 and who had high serum DDT levels in 1963. Reasoning that DDT use became widespread in 1945 and peaked around 1950, they concluded that the ages of 14–20 were a critical period in which DDT exposure leads to increased risk. This study, which suggests a connection between DDT exposure and breast cancer that would not be picked up by most studies, has received variable commentary in third party reviews. One review suggested that "previous studies that measured exposure in older women may have missed the critical period."[55][85] The National Toxicology Program notes that while the majority of studies have not found a relationship between DDT exposure and breast cancer that positive associations have been seen in a "few studies among women with higher levels of exposure and among certain subgroups of women"[70]
A 2015 case control study identified a link (odds ratio 3.4) between in-utero exposure (as estimated from archived maternal blood samples) and breast cancer diagnosis in daughters. The findings "support classification of DDT as an endocrine disruptor, a predictor of breast cancer, and a marker of high risk".[86]
Malaria control
Malaria remains the primary public health challenge in many countries. In 2015, there were 214 million cases of malaria worldwide resulting in an estimated 438,000 deaths, 90% of which occurred in Africa.[87] DDT is one of many tools to fight the disease. Its use in this context has been called everything from a "miracle weapon [that is] like Kryptonite to the mosquitoes,"[88] to "toxic colonialism".[89]
Before DDT, eliminating mosquito breeding grounds by drainage or poisoning with Paris green or pyrethrum was sometimes successful. In parts of the world with rising living standards, the elimination of malaria was often a collateral benefit of the introduction of window screens and improved sanitation.[34] A variety of usually simultaneous interventions represents best practice. These include antimalarial drugs to prevent or treat infection; improvements in public health infrastructure to diagnose, sequester and treat infected individuals; bednets and other methods intended to keep mosquitoes from biting humans; and vector control strategies[90] such as larvaciding with insecticides, ecological controls such as draining mosquito breeding grounds or introducing fish to eat larvae and indoor residual spraying (IRS) with insecticides, possibly including DDT. IRS involves the treatment of interior walls and ceilings with insecticides. It is particularly effective against mosquitoes, since many species rest on an indoor wall before or after feeding. DDT is one of 12 WHO–approved IRS insecticides.[37]
WHO's anti-malaria campaign of the 1950s and 1960s relied heavily on DDT and the results were promising, though temporary in developing countries. Experts tie malarial resurgence to multiple factors, including poor leadership, management and funding of malaria control programs; poverty; civil unrest; and increased irrigation. The evolution of resistance to first-generation drugs (e.g. chloroquine) and to insecticides exacerbated the situation.[20][91] Resistance was largely fueled by unrestricted agricultural use. Resistance and the harm both to humans and the environment led many governments to curtail DDT use in vector control and agriculture.[36] In 2006 WHO reversed a longstanding policy against DDT by recommending that it be used as an indoor pesticide in regions where malaria is a major problem.[92]
Once the mainstay of anti-malaria campaigns, as of 2008 only 12 countries used DDT, including India and some southern African states,[90] though the number was expected to rise.[20]
Initial effectiveness
When it was introduced in World War II, DDT was effective in reducing malaria morbidity and mortality.[30] WHO's anti-malaria campaign, which consisted mostly of spraying DDT and rapid treatment and diagnosis to break the transmission cycle, was initially successful as well. For example, in Sri Lanka, the program reduced cases from about one million per year before spraying to just 18 in 1963[93][94] and 29 in 1964. Thereafter the program was halted to save money and malaria rebounded to 600,000 cases in 1968 and the first quarter of 1969. The country resumed DDT vector control but the mosquitoes had evolved resistance in the interim, presumably because of continued agricultural use. The program switched to malathion, but despite initial successes, malaria continued its resurgence into the 1980s.[35][95]
DDT remains on WHO's list of insecticides recommended for IRS. After the appointment of Arata Kochi as head of its anti-malaria division, WHO's policy shifted from recommending IRS only in areas of seasonal or episodic transmission of malaria, to advocating it in areas of continuous, intense transmission.[96] WHO reaffirmed its commitment to phasing out DDT, aiming "to achieve a 30% cut in the application of DDT world-wide by 2014 and its total phase-out by the early 2020s if not sooner" while simultaneously combating malaria. WHO plans to implement alternatives to DDT to achieve this goal.[97]
South Africa continues to use DDT under WHO guidelines. In 1996, the country switched to alternative insecticides and malaria incidence increased dramatically. Returning to DDT and introducing new drugs brought malaria back under control.[98] Malaria cases increased in South America after countries in that continent stopped using DDT. Research data showed a strong negative relationship between DDT residual house sprayings and malaria. In a research from 1993 to 1995, Ecuador increased its use of DDT and achieved a 61% reduction in malaria rates, while each of the other countries that gradually decreased its DDT use had large increases.[52][99][100]
Mosquito resistance
In some areas resistance reduced DDT's effectiveness. WHO guidelines require that absence of resistance must be confirmed before using the chemical.[101] Resistance is largely due to agricultural use, in much greater quantities than required for disease prevention.
Resistance was noted early in spray campaigns. Paul Russell, former head of the Allied Anti-Malaria campaign, observed in 1956 that "resistance has appeared after six or seven years."[34] Resistance has been detected in Sri Lanka, Pakistan, Turkey and Central America and it has largely been replaced by organophosphate or carbamate insecticides, e.g. malathion or bendiocarb.[102]
In many parts of India, DDT is ineffective.[103] Agricultural uses were banned in 1989 and its anti-malarial use has been declining. Urban use ended.[104] One study concluded that "DDT is still a viable insecticide in indoor residual spraying owing to its effectivity in well supervised spray operation and high excito-repellency factor."[105]
Studies of malaria-vector mosquitoes in KwaZulu-Natal Province, South Africa found susceptibility to 4% DDT (WHO's susceptibility standard), in 63% of the samples, compared to the average of 87% in the same species caught in the open. The authors concluded that "Finding DDT resistance in the vector An. arabiensis, close to the area where we previously reported pyrethroid-resistance in the vector An. funestus Giles, indicates an urgent need to develop a strategy of insecticide resistance management for the malaria control programmes of southern Africa."[106]
DDT can still be effective against resistant mosquitoes[107] and the avoidance of DDT-sprayed walls by mosquitoes is an additional benefit of the chemical.[105] For example, a 2007 study reported that resistant mosquitoes avoided treated huts. The researchers argued that DDT was the best pesticide for use in IRS (even though it did not afford the most protection from mosquitoes out of the three test chemicals) because the others pesticides worked primarily by killing or irritating mosquitoes – encouraging the development of resistance.[107] Others argue that the avoidance behavior slows eradication.[108] Unlike other insecticides such as pyrethroids, DDT requires long exposure to accumulate a lethal dose; however its irritant property shortens contact periods. "For these reasons, when comparisons have been made, better malaria control has generally been achieved with pyrethroids than with DDT."[102] In India outdoor sleeping and night duties are common, implying that "the excito-repellent effect of DDT, often reported useful in other countries, actually promotes outdoor transmission."[109]
Residents' concerns
IRS is effective if at least 80% of homes and barns in a residential area are sprayed.[101] Lower coverage rates can jeopardize program effectiveness. Many residents resist DDT spraying, objecting to the lingering smell, stains on walls, and the potential exacerbation of problems with other insect pests.[102][108][110] Pyrethroid insecticides (e.g. deltamethrin and lambda-cyhalothrin) can overcome some of these issues, increasing participation.[102]
Human exposure
A 1994 study found that South Africans living in sprayed homes have levels that are several orders of magnitude greater than others.[55] Breast milk from South African mothers contains high levels of DDT and DDE.[55] It is unclear to what extent these levels arise from home spraying vs food residues. Evidence indicates that these levels are associated with infant neurological abnormalities.[102]
Most studies of DDT's human health effects have been conducted in developed countries where DDT is not used and exposure is relatively low.[38][55][111]
Illegal diversion to agriculture is also a concern as it is difficult to prevent and its subsequent use on crops is uncontrolled. For example, DDT use is widespread in Indian agriculture,[112] particularly mango production[113] and is reportedly used by librarians to protect books.[114] Other examples include Ethiopia, where DDT intended for malaria control is reportedly used in coffee production,[115] and Ghana where it is used for fishing."[116][117] The residues in crops at levels unacceptable for export have been an important factor in bans in several tropical countries.[102] Adding to this problem is a lack of skilled personnel and management.[108]
Criticism of restrictions on DDT use
A few people and groups have argued that limitations on DDT use for public health purposes have caused unnecessary morbidity and mortality from vector-borne diseases, with some claims of malaria deaths ranging as high as the hundreds of thousands[118] and millions.[119] Robert Gwadz of the US National Institutes of Health said in 2007, "The ban on DDT may have killed 20 million children."[120] These arguments were rejected as "outrageous" by former WHO scientist Socrates Litsios.[88] May Berenbaum, University of Illinois entomologist, says, "to blame environmentalists who oppose DDT for more deaths than Hitler is worse than irresponsible."[88]
Hans Herren & Charles Mbogo[121]
Criticisms of a DDT "ban" often specifically reference the 1972 United States ban (with the erroneous implication that this constituted a worldwide ban and prohibited use of DDT in vector control). Reference is often made to Silent Spring, even though Carson never pushed for a DDT ban. John Quiggin and Tim Lambert wrote, "the most striking feature of the claim against Carson is the ease with which it can be refuted."[122]
Investigative journalist Adam Sarvana and others characterize these notion as "myths" promoted principally by Roger Bate of the pro-DDT advocacy group Africa Fighting Malaria (AFM).[123][124]
Alternatives
Insecticides
Organophosphate and carbamate insecticides, e.g. malathion and bendiocarb, respectively, are more expensive than DDT per kilogram and are applied at roughly the same dosage. Pyrethroids such as deltamethrin are also more expensive than DDT, but are applied more sparingly (0.02–0.3 g/m2 vs 1–2 g/m2), so the net cost per house is about the same.[37]
Non-chemical vector control
Before DDT, malaria was successfully eliminated or curtailed in several tropical areas by removing or poisoning mosquito breeding grounds and larva habitats, for example by eliminating standing water. These methods have seen little application in Africa for more than half a century.[125] According to CDC, such methods are not practical in Africa because "Anopheles gambiae, one of the primary vectors of malaria in Africa, breeds in numerous small pools of water that form due to rainfall ... It is difficult, if not impossible, to predict when and where the breeding sites will form, and to find and treat them before the adults emerge."[126]
The relative effectiveness of IRS versus other malaria control techniques (e.g. bednets or prompt access to anti-malarial drugs) varies and is dependent on local conditions.[37]
A WHO study released in January 2008 found that mass distribution of insecticide-treated mosquito nets and artemisinin–based drugs cut malaria deaths in half in malaria-burdened Rwanda and Ethiopia. IRS with DDT did not play an important role in mortality reduction in these countries.[127][128]
Vietnam has enjoyed declining malaria cases and a 97% mortality reduction after switching in 1991 from a poorly funded DDT-based campaign to a program based on prompt treatment, bednets and pyrethroid group insecticides.[129]
In Mexico, effective and affordable chemical and non-chemical strategies were so successful that the Mexican DDT manufacturing plant ceased production due to lack of demand.[130]
A review of fourteen studies in sub-Saharan Africa, covering insecticide-treated nets, residual spraying, chemoprophylaxis for children, chemoprophylaxis or intermittent treatment for pregnant women, a hypothetical vaccine and changing front–line drug treatment, found decision making limited by the lack of information on the costs and effects of many interventions, the small number of cost-effectiveness analyses, the lack of evidence on the costs and effects of packages of measures and the problems in generalizing or comparing studies that relate to specific settings and use different methodologies and outcome measures. The two cost-effectiveness estimates of DDT residual spraying examined were not found to provide an accurate estimate of the cost-effectiveness of DDT spraying; the resulting estimates may not be good predictors of cost-effectiveness in current programs.[131]
However, a study in Thailand found the cost per malaria case prevented of DDT spraying (US$1.87) to be 21% greater than the cost per case prevented of lambda-cyhalothrin–treated nets (US$1.54),[132] casting some doubt on the assumption that DDT was the most cost-effective measure. The director of Mexico's malaria control program found similar results, declaring that it was 25% cheaper for Mexico to spray a house with synthetic pyrethroids than with DDT.[130] However, another study in South Africa found generally lower costs for DDT spraying than for impregnated nets.[133]
A more comprehensive approach to measuring cost-effectiveness or efficacy of malarial control would not only measure the cost in dollars, as well as the number of people saved, but would also consider ecological damage and negative human health impacts. One preliminary study found that it is likely that the detriment to human health approaches or exceeds the beneficial reductions in malarial cases, except perhaps in epidemics. It is similar to the earlier study regarding estimated theoretical infant mortality caused by DDT and subject to the criticism also mentioned earlier.[134]
A study in the Solomon Islands found that "although impregnated bed nets cannot entirely replace DDT spraying without substantial increase in incidence, their use permits reduced DDT spraying."[135]
A comparison of four successful programs against malaria in Brazil, India, Eritrea and Vietnam does not endorse any single strategy but instead states, "Common success factors included conducive country conditions, a targeted technical approach using a package of effective tools, data-driven decision-making, active leadership at all levels of government, involvement of communities, decentralized implementation and control of finances, skilled technical and managerial capacity at national and sub-national levels, hands-on technical and programmatic support from partner agencies, and sufficient and flexible financing."[136]
DDT resistant mosquitoes have generally proved susceptible to pyrethroids. Thus far, pyrethroid resistance in Anopheles has not been a major problem.[102]
See also
- DDT in Australia
- DDT in New Zealand
- DDT in the United States
- Mickey Slim, an alleged cocktail that combined gin with a pinch of DDT.
- Operation Cat Drop
- Biomagnification
References
- 1 2 3 4 5 6 7 8 9 10 11 12 Toxicological Profile: for DDT, DDE, and DDE. Agency for Toxic Substances and Disease Registry, September 2002.
- ↑ "NIOSH Pocket Guide to Chemical Hazards #0174". National Institute for Occupational Safety and Health (NIOSH).
- ↑ "DDT". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- ↑ "NIOSH Pocket Guide to Chemical Hazards".
- 1 2 NobelPrize.org: The Nobel Prize in Physiology of Medicine 1948, accessed July 26, 2007.
- 1 2 3 4 Conis, Elena (2017). "Beyond Silent Spring: An Alternate History of DDT". Distillations. 2 (4): 16–23. Retrieved 24 July 2017.
- 1 2 Lear, Linda (1 April 2009). Rachel Carson: Witness for Nature. Mariner Books. ISBN 978-0-547-23823-4.
- 1 2 Larson K (December 1, 2007). "Bad Blood". On Earth (Winter 2008). Retrieved June 5, 2008.
- ↑ Moyers B (September 21, 2007). "Rachel Carson and DDT". Retrieved March 5, 2011.
- 1 2 3 Stokstad E (June 2007). "Species conservation. Can the bald eagle still soar after it is delisted?". Science. 316 (5832): 1689–90. PMID 17588911. doi:10.1126/science.316.5832.1689.
- ↑ United States Fish and Wildlife Service, Fact Sheet: Natural History, Ecology, and History of Recovery
- 1 2 3 4 5 DDT and its derivatives, Environmental Health Criteria monograph No. 009, Geneva: World Health Organization, 1979, ISBN 92-4-154069-9
- 1 2 3 DDT and Its Derivatives: Environmental Aspects, Environmental Health Criteria monograph No. 83, Geneva: World Health Organization, ISBN 92-4-154283-7
- ↑ Geisz HN, Dickhut RM, Cochran MA, Fraser WR, Ducklow HW (June 2008). "Melting glaciers: a probable source of DDT to the Antarctic marine ecosystem". Environmental Science & Technology. 42 (11): 3958–62. Bibcode:2008EnST...42.3958G. PMID 18589951. doi:10.1021/es702919n.
- ↑ "Agribusiness, Biotechnology and War". Organicconsumers.org.
- ↑ David D (July 4, 2008). "McIntosh residents file suit against Ciba". Archived from the original on November 18, 2010. Retrieved July 7, 2008.
- ↑ Environmental Cleanup Site Information Database for Arkema (former Pennwalt) facility, Oregon DEQ, April 2009.
- ↑ Horvath R (January 27, 2008). "Tests shed light on how pCBSA got into St. Louis water". Morning Sun. Michigan, United States: Journal Register Company. Archived from the original on July 5, 2008. Retrieved May 16, 2008.
- 1 2 3 4 5 6 DDT Regulatory History: A Brief Survey (to 1975), U.S. EPA, July 1975.
- 1 2 3 4 van den Berg H (October 23, 2008). "Global status of DDT and its alternatives for use in vector control to prevent disease" (PDF). Stockholm Convention on Persistent Organic Pollutants/United Nations Environment Programme. Archived (PDF) from the original on November 18, 2010. Retrieved November 22, 2008.
- 1 2 "Report of the Third Expert Group Meeting on DDT". UNEP/POPS/DDT-EG.3/3, Stockholm Convention on Persistent Organic Pollutants. November 12, 2010.
- ↑ DeCarvalho Anderson, Juliana (3 May 2013). "DDT". Toxipedia. Retrieved 27 August 2016.
- ↑ Denholm I, Devine GJ, Williamson MS (September 2002). "Evolutionary genetics. Insecticide resistance on the move". Science. 297 (5590): 2222–23. PMID 12351778. doi:10.1126/science.1077266.
- ↑ Pedra JH, McIntyre LM, Scharf ME, Pittendrigh BR (May 2004). "Genome-wide transcription profile of field- and laboratory-selected dichlorodiphenyltrichloroethane (DDT)-resistant Drosophila". Proceedings of the National Academy of Sciences of the United States of America. 101 (18): 7034–39. Bibcode:2004PNAS..101.7034P. PMC 406461
 . PMID 15118106. doi:10.1073/pnas.0400580101.
. PMID 15118106. doi:10.1073/pnas.0400580101. - ↑ Othmar Zeidler (1874). "Verbindungen von Chloral mit Brom- und Chlorbenzol" [Compounds of chloral with bromo- and chlorobenzene]. Berichte der Deutschen Chemischen Gesellschaft. 7 (2): 1180–81. doi:10.1002/cber.18740070278. On p. 1181, Zeidler called DDT dimonochlorphenyltrichloräthan.
- ↑ Augustin F (1993). Zur Geschichte des Insektizids Dichlordiphenyltrichloräthan (DDT) unter besonderer Berücksichtigung der Leistung des Chemikers Paul Müller (1899–1965). Leipzig: Medizinische Fakultät der Universität Leipzig. pp. 1–77.
- ↑ Brand K, Bausch W (1930). "Über Verbindungen der Tetraaryl-butanreihe. 10. Mitteilung. Über die Reduktion organischer Halogenverbindungen und Über Verbindungen der Tetraaryl-butanreihe". Journal für Praktische Chemie. 127: 219–39. doi:10.1002/prac.19301270114.
- ↑ Brand K, Horn O, Bausch W (1930). "Die elektrochemische Darstellung von 1,1,4,4-p,p′,p",p‴-Tetraphenetyl-butin-2 und von 1,1,4,4-p,p′,p",p‴-Tetra(chlorphenyl)-butin-2. 11. Mitteilung. Über die Reduktion organischer Halogenverbindungen und Verbindungen der Tetraarylbutanreihe". Journal für Praktische Chemie. 127: 240–47. doi:10.1002/prac.19301270115.
- ↑ Wolfgang von Leuthold, Schädlingsbekämpfung. DRP Nr 673246, April 27, 1934
- 1 2 3 Dunlap, Thomas (14 July 2014). DDT: Scientists, Citizens, and Public Policy. Princeton University Press. ISBN 978-1-4008-5385-4.
- ↑ de Zulueta J (June 1998). "The end of malaria in Europe: an eradication of the disease by control measures". Parassitologia. 40 (1–2): 245–46. PMID 9653750.
- ↑ "CDC – Malaria – About Malaria – History – Elimination of Malaria in the United States (1947–1951)".
- ↑ Mendis K, Rietveld A, Warsame M, Bosman A, Greenwood B, Wernsdorfer WH (July 2009). "From malaria control to eradication: The WHO perspective". Tropical Medicine & International Health. 14 (7): 802–09. PMID 19497083. doi:10.1111/j.1365-3156.2009.02287.x.
- 1 2 3 Gladwell M (July 2, 2001). "The Mosquito Killer". The New Yorker.
- 1 2 Harrison GA (1 June 1978). Mosquitoes, Malaria, and Man: A History of the Hostilities Since 1880. Dutton. ISBN 978-0-525-16025-0.
- 1 2 3 Chapin G, Wasserstrom R (1981). "Agricultural production and malaria resurgence in Central America and India". Nature. 293 (5829): 181–85. PMID 7278974. doi:10.1038/293181a0.
- 1 2 3 4 5 Sadasivaiah S, Tozan Y, Breman JG (December 2007). "Dichlorodiphenyltrichloroethane (DDT) for indoor residual spraying in Africa: how can it be used for malaria control?". The American Journal of Tropical Medicine and Hygiene. 77 (6 Suppl): 249–63. PMID 18165500.
- 1 2 3 4 5 Rogan WJ, Chen A (2005). "Health risks and benefits of bis(4-chlorophenyl)-1,1,1-trichloroethane (DDT)". Lancet. 366 (9487): 763–73. PMID 16125595. doi:10.1016/S0140-6736(05)67182-6.
- ↑ Davis, Frederick Rowe (2014). Banned : a history of pesticides and the science of toxicology. [S.l.]: Yale University Press. p. 26. ISBN 978-0300205176. Retrieved 25 July 2017.
- ↑ Conis, Elena (October 28, 2016). "DDT Disbelievers: Health and the New Economic Poisons in Georgia after World War II". Southern Spaces. Retrieved 25 July 2017.
- ↑ Greenberg DS (May 1963). "Pesticides: White House Advisory Body Issues Report Recommending Steps to Reduce Hazard to Public". Science. 140 (3569): 878–79. PMID 17810673. doi:10.1126/science.140.3569.878. Archived from the original on June 28, 2009.
- ↑ Michaels D (2008). Doubt is Their Product: How Industry's Assault on Science Threatens Your Health. New York: Oxford University Press. ISBN 978-0-19-530067-3.
- ↑ "Sue the Bastards". TIME. October 18, 1971. Archived from the original on November 18, 2010.
- ↑ "AEI – Short Publications – The Rise, Fall, Rise, and Imminent Fall of DDT". Archived from the original on November 18, 2010.
- ↑ "Selected passages from the history of the Hungarian plant protection administration on the 50th anniversary of establishing the county plant protection stations".
- ↑ "DDT, Decision Guidance Document, Joint FAO/UNEP Programme for the operation of Prior Informed Consent, UNEP/FAO, Rome, Italy, 1991." (PDF).
- ↑ "Stockholm Convention on Persistent Organic Pollutants." (PDF).
- ↑ "WHO. Strengthening malaria control while reducing reliance on DDT. 2011.".
- ↑ "MFI second page". Malaria Foundation International. Archived from the original on November 18, 2010. Retrieved March 15, 2006.
- ↑ "Concern over excessive DDT use in Jiribam fields". The Imphal Free Press. May 5, 2008. Archived from the original on December 6, 2008. Retrieved May 5, 2008.
- ↑ "Is DDT still effective and needed in malaria control?". Malaria Foundation International. Archived from the original on November 18, 2010. Retrieved March 15, 2006.
- 1 2 Roberts DR, Laughlin LL, Hsheih P, Legters LJ (July–September 1997). "DDT, global strategies, and a malaria control crisis in South America". Emerging Infectious Diseases. 3 (3): 295–302. PMC 2627649
 . PMID 9284373. doi:10.3201/eid0303.970305.
. PMID 9284373. doi:10.3201/eid0303.970305. - ↑ "The Grasshopper Effect and Tracking Hazardous Air Pollutants". The Science and the Environment Bulletin. Environment Canada (May/June 1998). Archived from the original on September 28, 2004.
- ↑ Connell, D.; et al. (1999). Introduction to Ecotoxicology. Blackwell Science. p. 68. ISBN 0-632-03852-7.
- 1 2 3 4 5 6 7 8 9 Eskenazi B, Chevrier J, Rosas LG, Anderson HA, Bornman MS, Bouwman H, Chen A, Cohn BA, de Jager C, Henshel DS, Leipzig F, Leipzig JS, Lorenz EC, Snedeker SM, Stapleton D (September 2009). "The Pine River statement: human health consequences of DDT use". Environmental Health Perspectives. 117 (9): 1359–67. PMC 2737010
 . PMID 19750098. doi:10.1289/ehp.11748.
. PMID 19750098. doi:10.1289/ehp.11748. - ↑ USDA, Pesticide Data Program Annual Summary Calendar YearPesticide Data Program Annual Summary Calendar Year 2005, November 2006.
- ↑ Vos JG, Dybing E, Greim HA, Ladefoged O, Lambré C, Tarazona JV, Brandt I, Vethaak AD (January 2000). "Health effects of endocrine-disrupting chemicals on wildlife, with special reference to the European situation". Critical Reviews in Toxicology. 30 (1): 71–133. PMID 10680769. doi:10.1080/10408440091159176.
- 1 2 Lundholm CD (October 1997). "DDE-induced eggshell thinning in birds: effects of p,p'-DDE on the calcium and prostaglandin metabolism of the eggshell gland". Comparative Biochemistry and Physiology C. 118 (2): 113–28. PMID 9490182. doi:10.1016/S0742-8413(97)00105-9.
- 1 2 Tubbs, Christopher W. (2016). "California condors and DDT: Examining the effects of endocrine disrupting chemicals in a critically endangered species". Endocrine Disruptors. 4: e1173766. doi:10.1080/23273747.2016.1173766.
- 1 2 Snyder, Noel F. R.; Meretsky, Vicky J. (2002). "California Condors and DDE: A re-evaluation". Ibis. 145 (1): 136–51. doi:10.1046/j.1474-919X.2003.00132.x.
- ↑ "Endangered and Threatened Wildlife and Plants; 12-Month Petition Finding and Proposed Rule To Remove the Brown Pelican (Pelecanus occidentalis) From the Federal List of Endangered and Threatened Wildlife; Proposed Rule," Fish and Wildlife Service, U.S. Department of the Interior, February 20, 2008. 73 FR 9407
- ↑ Moir, John, "New Hurdle for California Condors May Be DDT From Years Ago", The New York Times, November 15, 2010. Retrieved November 15, 2010.
- ↑ Kurle CM, Bakker VJ, Copeland H, Burnett J, Jones Scherbinski J, Brandt J, Finkelstein ME (2016). "Terrestrial Scavenging of Marine Mammals: Cross-Ecosystem Contaminant Transfer and Potential Risks to Endangered California Condors (Gymnogyps californianus)". Environmental Science & Technology. 50 (17): 9114–23. PMID 27434394. doi:10.1021/acs.est.6b01990.
- ↑ Walker C, Sibly RM, Hopkin S, Peakall DB (22 December 2005). Principles of ecotoxicology (3rd ed.). Boca Raton, FL: CRC/Taylor & Francis. pp. 300–. ISBN 978-0-8493-3635-5.
- ↑ Guillette LJ (2006). "Endocrine Disrupting Contaminants" (PDF). Archived (PDF) from the original on November 18, 2010. Retrieved February 2, 2007.
- ↑ Holm L, Blomqvist A, Brandt I, Brunström B, Ridderstråle Y, Berg C (October 2006). "Embryonic exposure to o,p'-DDT causes eggshell thinning and altered shell gland carbonic anhydrase expression in the domestic hen". Environmental Toxicology and Chemistry / SETAC. 25 (10): 2787–93. PMID 17022422. doi:10.1897/05-619R.1.
- ↑ "Endocrine (Hormone) Disruptors". United States Fish and Wildlife Service. Retrieved 8 April 2015.
- ↑ "Endocrine Disruptors" (PDF). National Institute of Environmental Health Sciences. 2007. Retrieved April 2015. Check date values in:
|access-date=(help) - ↑ "European Food Safety Administration – DDT" (PDF). Retrieved 2014-10-29.
- 1 2 "DDT" (PDF). National Toxicology Program. Retrieved 2014-10-29.
- ↑ "IARC - DDT" (PDF). Retrieved 2014-10-29.
- ↑ Hejmej, Anna; Kotula-Balak, Magorzata; Bilinsk, Barbara (2011). "Antiandrogenic and Estrogenic Compounds: Effect on Development and Function of Male Reproductive System". Steroids – Clinical Aspect. doi:10.5772/28538.
- 1 2 Cohn BA, Wolff MS, Cirillo PM, Sholtz RI (October 2007). "DDT and breast cancer in young women: new data on the significance of age at exposure". Environmental Health Perspectives. 115 (10): 1406–14. PMC 2022666
 . PMID 17938728. doi:10.1289/ehp.10260.
. PMID 17938728. doi:10.1289/ehp.10260. - ↑ "DDT, p,p' - toxicity, ecological toxicity and regulatory information".
- ↑ World Health Organization, The WHO Recommended Classification of Pesticides by Hazard, 2005.
- 1 2 3 Agarwal, Ashok; Aponte-Mellado, Anamar; Premkumar, Beena J; Shaman, Amani; Gupta, Sajal (2012). "The effects of oxidative stress on female reproduction: a review". Reproductive Biology and Endocrinology. 10 (1): 49. doi:10.1186/1477-7827-10-49.
In general, incidental human exposure to DDT has been considered relatively non-toxic, but prolonged exposure has long been recognized to adversely affect reproduction.
- ↑ Jurewicz J, Hanke W, Radwan M, Bonde JP (January 2010). "Environmental factors and semen quality". International Journal of Occupational Medicine and Environmental Health. 22 (4): 305–29. PMID 20053623. doi:10.2478/v10001-009-0036-1.
- ↑ Eskenazi, Brenda; Chevrier, Jonathan; Rosas, Lisa Goldman; Anderson, Henry A.; Bornman, Maria S.; Bouwman, Henk; Chen, Aimin; Cohn, Barbara A.; de Jager, Christiaan; Henshel, Diane S.; Leipzig, Felicia; Leipzig, John S.; Lorenz, Edward C.; Snedeker, Suzanne M.; Stapleton, Darwin (September 2009). "The Pine River Statement: Human Health Consequences of DDT Use". Environmental Health Perspectives. 117 (9): 1359–67. doi:10.1289/ehp.11748.
Overall, the few studies conducted to date suggest that DDT exposure may affect time to pregnancy, but more research is needed.
- ↑ Chevrier J, Eskenazi B, Holland N, Bradman A, Barr DB (August 2008). "Effects of exposure to polychlorinated biphenyls and organochlorine pesticides on thyroid function during pregnancy". American Journal of Epidemiology. 168 (3): 298–310. PMC 2727265
 . PMID 18550560. doi:10.1093/aje/kwn136.
. PMID 18550560. doi:10.1093/aje/kwn136. - ↑ IARC Monographs evaluate DDT, lindane, and 2,4-D
- ↑ http://link.springer.com/article/10.1007%2Fs10552-017-0854-6
- ↑ Park JH, Cha ES, Ko Y, Hwang MS, Hong JH, Lee WJ (April 2014). "Exposure to Dichlorodiphenyltrichloroethane and the Risk of Breast Cancer: A Systematic Review and Meta-analysis". Osong Public Health and Research Perspectives. 5 (2): 77–84. PMC 4064641
 . PMID 24955316. doi:10.1016/j.phrp.2014.02.001.
. PMID 24955316. doi:10.1016/j.phrp.2014.02.001. - ↑ Ingber SZ, Buser MC, Pohl HR, Abadin HG, Murray HE, Scinicariello F (December 2013). "DDT/DDE and breast cancer: a meta-analysis". Regulatory Toxicology and Pharmacology. 67 (3): 421–33. PMID 24021539. doi:10.1016/j.yrtph.2013.08.021.
- ↑ Smith-Bindman R (July 2012). "Environmental causes of breast cancer and radiation from medical imaging: findings from the Institute of Medicine report". Archives of Internal Medicine. 172 (13): 1023–27. PMC 3936791
 . PMID 22688684. doi:10.1001/archinternmed.2012.2329.
. PMID 22688684. doi:10.1001/archinternmed.2012.2329. - ↑ Clapp RW, Jacobs MM, Loechler EL (2008). "Environmental and occupational causes of cancer: new evidence 2005-2007". Reviews on Environmental Health. 23 (1): 1–37. PMC 2791455
 . PMID 18557596. doi:10.1515/REVEH.2008.23.1.1.
. PMID 18557596. doi:10.1515/REVEH.2008.23.1.1. - ↑ Cohn BA, La Merrill M, Krigbaum NY, Yeh G, Park JS, Zimmermann L, Cirillo PM (August 2015). "DDT Exposure in Utero and Breast Cancer". The Journal of Clinical Endocrinology and Metabolism. 100 (8): 2865–72. PMC 4524999
 . PMID 26079774. doi:10.1210/jc.2015-1841.
. PMID 26079774. doi:10.1210/jc.2015-1841. - ↑ "Malaria Fact sheet N°94". WHO. Retrieved 2 February 2016.
- 1 2 3 Weir, Kirsten (June 29, 2007). "Rachel Carson's birthday bashing". Salon.com. Retrieved July 1, 2007.
- ↑ Paull, John (November 3, 2007). "Toxic Colonialism". New Scientist (2628): 25.
- 1 2 2009 WHO World Malaria Report 2009
- ↑ Feachem RG, Sabot OJ (May 2007). "Global malaria control in the 21st century: a historic but fleeting opportunity". JAMA. 297 (20): 2281–84. PMID 17519417. doi:10.1001/jama.297.20.2281.
- ↑ "WHO Urges Use of DDT in Africa". Washington Post. September 16, 2006.
- ↑ Garrett, Laurie (31 October 1994). The Coming Plague: Newly Emerging Diseases in a World Out of Balance. Farrar, Straus and Giroux. p. 51. ISBN 978-1-4299-5327-6.
- ↑ Malaria: A Disease Close to Eradication Grows, Aided by Political Tumult in Sri Lanka, Donald G. McNeil Jr, The New York Times, December 27, 2010.
- ↑ Karunaweera ND, Galappaththy GN, Wirth DF (2014). "On the road to eliminate malaria in Sri Lanka: lessons from history, challenges, gaps in knowledge and research needs". Malaria Journal. 13: 59. PMC 3943480
 . PMID 24548783. doi:10.1186/1475-2875-13-59.
. PMID 24548783. doi:10.1186/1475-2875-13-59. - ↑ WHO |WHO gives indoor use of DDT a clean bill of health for controlling malaria
- ↑ "WHO – Countries move toward more sustainable ways to roll back malaria".
- ↑ Yamey G (May 2004). "Roll Back Malaria: a failing global health campaign". BMJ. 328 (7448): 1086–87. PMC 406307
 . PMID 15130956. doi:10.1136/bmj.328.7448.1086.
. PMID 15130956. doi:10.1136/bmj.328.7448.1086. - ↑ Griffing SM, Gamboa D, Udhayakumar V (2013). "The history of 20th century malaria control in Peru". Malaria Journal. 12: 303. PMC 3766208
 . PMID 24001096. doi:10.1186/1475-2875-12-303.
. PMID 24001096. doi:10.1186/1475-2875-12-303. - ↑ Curtis CF (December 2002). "Should the use of DDT be revived for malaria vector control?". Biomédica. 22 (4): 455–61. PMID 12596442.
- 1 2 Indoor Residual Spraying: Use of Indoor Residual Spraying for Scaling Up Global Malaria Control and Elimination. World Health Organization, 2006.
- 1 2 3 4 5 6 7 C.F. Curtis, Control of Malaria Vectors in Africa and Asia
- ↑ Sharma VP (September 1999). "Current scenario of malaria in India". Parassitologia. 41 (1–3): 349–53. PMID 10697882.
- ↑ Agarwal R (May 2001). "No Future in DDT: A case study of India". Pesticide Safety News.
- 1 2 Sharma SN, Shukla RP, Raghavendra K, Subbarao SK (June 2005). "Impact of DDT spraying on malaria transmission in Bareilly District, Uttar Pradesh, India". Journal of Vector Borne Diseases. 42 (2): 54–60. PMID 16161701.
- ↑ Hargreaves K, Hunt RH, Brooke BD, Mthembu J, Weeto MM, Awolola TS, Coetzee M (December 2003). "Anopheles arabiensis and An. quadriannulatus resistance to DDT in South Africa". Medical and Veterinary Entomology. 17 (4): 417–22. PMID 14651656. doi:10.1111/j.1365-2915.2003.00460.x.
- 1 2 Grieco JP, Achee NL, Chareonviriyaphap T, Suwonkerd W, Chauhan K, Sardelis MR, Roberts DR (2007). Krishna S, ed. "A new classification system for the actions of IRS chemicals traditionally used for malaria control". PLoS ONE. 2 (8): e716. Bibcode:2007PLoSO...2..716G. PMC 1934935
 . PMID 17684562. doi:10.1371/journal.pone.0000716.
. PMID 17684562. doi:10.1371/journal.pone.0000716. 
- 1 2 3 Mabaso ML, Sharp B, Lengeler C (August 2004). "Historical review of malarial control in southern African with emphasis on the use of indoor residual house-spraying". Tropical Medicine & International Health. 9 (8): 846–56. PMID 15303988. doi:10.1111/j.1365-3156.2004.01263.x.
- ↑ Sharma VP (December 2003). "DDT: The fallen angel" (PDF). Current Science. 85 (11): 1532–37.
- ↑ In Malaria War, South Africa Turns To Pesticide Long Banned in the West, Roger Thurow, Wall Street Journal, July 26, 2001
- ↑ Science Daily (May 9, 2009). "Unprecedented Use Of DDT Concerns Experts". ScienceDaily.com. Retrieved May 30, 2009.
- ↑ Jayashree J (10 June 2009). "Pesticide level in veggies, fruits rises". Economic Times. Retrieved June 10, 2009.
- ↑ Sanjana (June 13, 2009). "A Whole Fruit". Tehelka Magazine. 6 (23).
- ↑ Chakravartty A (8 June 2009). "State public libraries gasp for breath". Indian Express. Retrieved June 8, 2009.
- ↑ Katima J (June 2009). "African NGOs outline commitment to malaria control without DDT" (PDF). Pesticides News (84): 5.
- ↑ Ghana News Agency (November 17, 2009). "Ministry moves to check unorthodox fishing methods". Ghana News Agency. Archived from the original on November 18, 2010. Retrieved November 18, 2009.
- ↑ Appiah S (27 April 2010). "Northern fisherfolks complain of committee’s harassment". Joy Online. Retrieved April 27, 2010.
- ↑ Kristof ND (March 12, 2005). "I Have a Nightmare". New York Times. A-15.
- ↑ Souder, William (September 4, 2012). "Rachel Carson Didn’t Kill Millions of Africans". Slate. Retrieved September 5, 2012.
- ↑ Finkel, Michael (July 2007). "Malaria". National Geographic.
- ↑ Herren, Hans Rudolf; Mbogo, Charles (2010). "The Role of DDT in Malaria Control". Environmental Health Perspectives. Environmental Health Perspectives. 118 (7): a282. doi:10.1289/ehp.1002279. Retrieved 2017-02-06.
- ↑ Quiggin J, Lambert T (May 2008). "Rehabilitating Carson". Prospect.
- ↑ Sarvana A (May 28, 2009). "Bate and Switch: How a free-market magician manipulated two decades of environmental science". Natural Resources New Service. Archived from the original on May 24, 2010. Retrieved June 2, 2009.
- ↑ Gutstein D (2009). Not a Conspiracy Theory: How Business Propaganda is Hijacking Democracy. ISBN 978-1-55470-191-9.. Relevant excerpt at Gutstein D (January 22, 2010). "Inside the DDT Propaganda Machine". The Tyee. Retrieved January 22, 2010.
- ↑ Killeen GF, Fillinger U, Kiche I, Gouagna LC, Knols BG (October 2002). "Eradication of Anopheles gambiae from Brazil: lessons for malaria control in Africa?". The Lancet. Infectious Diseases. 2 (10): 618–27. PMID 12383612. doi:10.1016/S1473-3099(02)00397-3.
- ↑ "CDC – Malaria – Malaria Worldwide – How Can Malaria Cases and Deaths Be Reduced? – Larval Control and Other Vector Control Interventions".
- ↑ Impact of long-lasting insecticidal-treated nets (LLINs) and artemisinin-based combination therapies (ACTs) measured using surveillance data in four African countries. World Health Organization, January 31, 2008.
- ↑ Malaria deaths halved in Rwanda and Ethiopia Better drugs, mosquito nets are the crucial tools, David Brown (Washington Post), SF Chronicle, A-12, February 1, 2008.
- ↑ World Health Organization, "A story to be shared: The successful fight against malaria in Vietnam," November 6, 2000. Archived February 26, 2008, at the Wayback Machine.
- 1 2 "DDT & Malaria" (PDF). Archived (PDF) from the original on May 21, 2010. Retrieved March 11, 2009.
- ↑ Goodman CA, Mills AJ (December 1999). "The evidence base on the cost-effectiveness of malaria control measures in Africa" (PDF). Health Policy and Planning. 14 (4): 301–12. PMID 10787646. doi:10.1093/heapol/14.4.301.
- ↑ Kamolratanakul P, Butraporn P, Prasittisuk M, Prasittisuk C, Indaratna K (October 2001). "Cost-effectiveness and sustainability of lambdacyhalothrin-treated mosquito nets in comparison to DDT spraying for malaria control in western Thailand". The American Journal of Tropical Medicine and Hygiene. 65 (4): 279–84. PMID 11693869.
- ↑ Goodman CA, Mnzava AE, Dlamini SS, Sharp BL, Mthembu DJ, Gumede JK (April 2001). "Comparison of the cost and cost-effectiveness of insecticide-treated bednets and residual house-spraying in KwaZulu-Natal, South Africa". Tropical Medicine & International Health. 6 (4): 280–95. PMID 11348519. doi:10.1046/j.1365-3156.2001.00700.x.
- ↑ Corin S, Weaver S (2005). "A risk analysis model with an ecological perspective on DDT and malaria control in South Africa" (PDF). Journal of Rural and Tropical Public Health. 4 (4): 21–32.
- ↑ Over M, Bakote'e B, Velayudhan R, Wilikai P, Graves PM (August 2004). "Impregnated nets or DDT residual spraying? Field effectiveness of malaria prevention techniques in solomon islands, 1993–1999". The American Journal of Tropical Medicine and Hygiene. 71 (2 Suppl): 214–23. PMID 15331840.
- ↑ Barat LM (January 2006). "Four malaria success stories: how malaria burden was successfully reduced in Brazil, Eritrea, India, and Vietnam". The American Journal of Tropical Medicine and Hygiene. 74 (1): 12–16. PMID 16407339.
Further reading
- David Kinkela. DDT and the American Century: Global Health, Environmental Politics, and the Pesticide That Changed the World (University of North Carolina Press, 2011).
External links
| Wikimedia Commons has media related to DDT. |
- Chemistry
- DDT at The Periodic Table of Videos (University of Nottingham)
- Toxicity
- "DDT Technical Fact Sheet" (PDF). National Pesticide Information Center.
- "DDT General Fact Sheet" (PDF). National Pesticide Information Center.
- "DDT". Pesticide Information Profiles. EXTOXNET.
- Scorecard: The Pollution Information Site – DDT
- Interview with Barbara Cohn, PhD about DDT and breast cancer
- Pesticide residues in food 2000 : DDT
- "DDT". NIOSH Pocket Guide to Chemical Hazards. CDC.
- Politics and DDT
- Bailey, Ronald (7 January 2004). "DDT, Eggshells, and Me". Reason magazine.
- Swartz, Aaron (September–October 2007). "Rachel Carson, Mass Murderer?: The creation of an anti-environmental myth". Extra!.
- Malaria and DDT
- Berenbaum, May (4 June 2005). "If Malaria's the Problem, DDT's Not the Only Answer". Washington Post.
- 'Andrew Spielman, Harvard School of Public Health, discusses environmentally friendly control of Malaria and uses of DDT Freeview video provided by the Vega Science Trust
- "Ugandan farmers push for DDT ban". ABC News. Australian Broadcasting Commission. 31 May 2008.
- DDT in Popular Culture

