Dexmethylphenidate
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Focalin, Focalin XR, Attenade, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603014 |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 11 – 52% |
| Protein binding | 30% |
| Metabolism | Liver |
| Biological half-life | 4 hours |
| Excretion | Kidney |
| Identifiers | |
| |
| Synonyms | d-threo-methylphenidate (D-TMP) |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C14H19NO2 |
| Molar mass | 233.31 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Dexmethylphenidate, sold under the trade names Focalin among others, is a central nervous system (CNS) stimulant used in the treatment of attention deficit hyperactivity disorder (ADHD) and narcolepsy.[1]
It is in the phenethylamine and piperidine classes of medications. It is the active dextrorotatory enantiomer of methylphenidate.
Medical uses
Dexmethylphenidate is used as a treatment for ADHD, usually along with psychological, educational, behavioral or other forms of treatment. It is proposed that stimulants help ameliorate the symptoms of ADHD by making it easier for the user to concentrate, avoid distraction, and control behavior. Placebo-controlled trials have shown that once-daily dexmethylphenidate XR was effective and generally well tolerated.[1] Improvements in ADHD symptoms in children were significantly greater for dexmethylphenidate XR versus placebo.[1] It also showed greater efficacy than osmotic controlled-release oral delivery system (OROS) methylphenidate over the first half of the laboratory classroom day but assessments late in the day favoured OROS methylphenidate.[1]
Contraindications
Methylphenidate is contraindicated for individuals using monoamine oxidase inhibitors (e.g., phenelzine and tranylcypromine), or individuals with agitation, tics, or glaucoma, or a hypersensitivity to any ingredients contained in methylphenidate pharmaceuticals.[2]
The US FDA gives methylphenidate a pregnancy category of C, and women are advised to only use the drug if the benefits outweigh the potential risks.[3] Not enough animal and human studies have been conducted to conclusively demonstrate an effect of methylphenidate on fetal development. In 2007, empirical literature included 63 cases of prenatal exposure to methylphenidate across three empirical studies.[4]
Adverse effects
Products containing dexmethylphenidate have a side effect profile comparable to those containing methylphenidate.[5]
Methylphenidate is generally well tolerated.[6][7] The most commonly observed adverse effects with a frequency greater than placebo include appetite loss, dry mouth, anxiety/nervousness, nausea, and insomnia. Gastrointestinal adverse effects may include abdominal pain and weight loss. Nervous system adverse effects may include akathisia (agitation/restlessness), irritability, dyskinesia (tics), lethargy (drowsiness/fatigue), and dizziness. Cardiac adverse effects may include palpitations, changes in blood pressure and heart rate (typically mild), tachycardia (rapid resting heart rate), and Raynaud's phenomenon (reduced blood flow to the hands and feet).[8] Ophthalmologic adverse effects may include blurred vision and dry eyes, with less frequent reports of diplopia and mydriasis.[9] Other adverse effects may include depression, emotional lability, confusion, and bruxism. Hyperhidrosis (increased sweating) is common. Chest pain is rarely observed.[10]
There is some evidence of mild reductions in growth rate with prolonged treatment in children, but no causal relationship has been established and reductions do not appear to persist long-term.[11] Hypersensitivity (including skin rash, urticaria, and fever) is sometimes reported. The Daytrana patch has a much higher rate of dermal reactions than oral methylphenidate.[12]
Methylphenidate can worsen psychosis in psychotic patients, and in very rare cases it has been associated with the emergence of new psychotic symptoms.[13] It should be used with extreme caution in patients with bipolar disorder due to the potential induction of mania or hypomania.[14] There have been very rare reports of suicidal ideation, but evidence does not support a link.[11] Logorrhea is occasionally reported. Libido disorders, disorientation, and hallucinations are very rarely reported. Priapism is a very rare adverse event that can be potentially serious.[15]
USFDA-commissioned studies from 2011 indicate that in children, young adults, and adults there is no association between serious adverse cardiovascular events (sudden death, heart attack, and stroke) and the medical use of methylphenidate or other ADHD stimulants.[16]
Because some adverse effects may only emerge during chronic use of methylphenidate, a constant watch for adverse effects is recommended.[17]
Overdose
The symptoms of a moderate acute overdose on methylphenidate primarily arise from central nervous system overstimulation; these symptoms include: vomiting, agitation, tremors, hyperreflexia, muscle twitching, euphoria, confusion, hallucinations, delirium, hyperthermia, sweating, flushing, headache, tachycardia, heart palpitations, cardiac arrhythmias, hypertension, mydriasis, and dryness of mucous membranes.[18][19] A severe overdose may involve symptoms such as hyperpyrexia, sympathomimetic toxidrome, convulsions, paranoia, stereotypy (a repetitive movement disorder), rapid muscle breakdown, coma, and circulatory collapse.[18][19][20] A methylphenidate overdose is rarely fatal with appropriate care.[20] Severe toxic reactions involving abscess and necrosis have been reported following injection of methylphenidate tablets into an artery.[21]
Treatment of a methylphenidate overdose typically involves the application of benzodiazepines, with antipsychotics, α-adrenoceptor agonists, and propofol serving as second-line therapies.[20]
Addiction and dependence
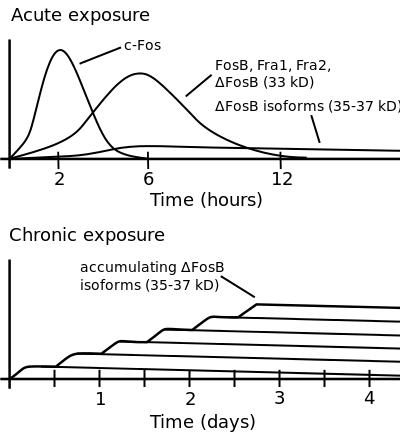
ΔFosB accumulation from excessive drug use
Top: this depicts the initial effects of high dose exposure to an addictive drug on gene expression in the nucleus accumbens for various genes in the Fos family.
Bottom: this illustrates the progressive increase in ΔFosB expression in the nucleus accumbens following repeated twice daily drug binges, where these phosphorylated (35–37 kD) ΔFosB isoforms persist in the D1-type medium spiny neurons of the nucleus accumbens for up to 2 months.[22][23] |
Pharmacological texts describe methylphenidate as a stimulant with effects, addiction liability, and dependence liability similar to amphetamine, a compound with moderate liability among addictive drugs;[24][25] accordingly, addiction and psychological dependence are possible and likely when methylphenidate is used at high doses as a recreational drug.[25][26] When used above the medical dose range, stimulants are associated with the development of stimulant psychosis.[27] As with all addictive drugs, the overexpression of ΔFosB in D1-type medium spiny neurons in the nucleus accumbens is implicated in methylphenidate addiction.[26][28]
Methylphenidate has shown some benefits as a replacement therapy for individuals who are addicted to and dependent upon methamphetamine.[29] Methylphenidate and amphetamine have been investigated as a chemical replacement for the treatment of cocaine addiction[30][31][32][33] in the same way that methadone is used as a replacement drug for physical dependence upon heroin. Its effectiveness in treatment of cocaine or psychostimulant addiction or psychological dependence has not been proven and further research is needed.[34]
Biomolecular mechanisms
Methylphenidate has the potential to induce euphoria due to its pharmacodynamic effect (i.e., dopamine reuptake inhibition) in the brain's reward system.[28] At therapeutic doses, ADHD stimulants do not sufficiently activate the reward system, or the reward pathway in particular, to the extent necessary to cause persistent increases in ΔFosB gene expression in the D1-type medium spiny neurons of the nucleus accumbens;[25][28][35] consequently, when taken as directed in doses that are commonly prescribed for the treatment of ADHD, methylphenidate use lacks the capacity to cause an addiction.[25][28][35] However, when methylphenidate is used at sufficiently high recreational doses through a bioavailable route of administration (e.g., insufflation or intravenous administration), particularly for use of the drug as a euphoriant, ΔFosB accumulates in the nucleus accumbens.[25][28] Hence, like any other addictive drug, regular recreational use of methylphenidate at high doses eventually gives rise to ΔFosB overexpression in D1-type neurons which subsequently triggers a series of gene transcription-mediated signaling cascades that induce an addiction.[28][35][36]
Interactions
Methylphenidate may inhibit the metabolism of coumarin anticoagulants, certain anticonvulsants, and some antidepressants (tricyclic antidepressants and selective serotonin reuptake inhibitors). Concomitant administration may require dose adjustments, possibly assisted by monitoring of plasma drug concentrations.[7] There are several case reports of methylphenidate inducing serotonin syndrome with concomitant administration of antidepressants.[37][38][39][40]
When methylphenidate is coingested with ethanol, a metabolite called ethylphenidate is formed via hepatic transesterification,[41][42] not unlike the hepatic formation of cocaethylene from cocaine and alcohol. The reduced potency of ethylyphenidate and its minor formation means it does not contribute to the pharmacological profile at therapeutic doses and even in overdose cases ethylphenidate concentrations remain negligible.[43][44]
Coingestion of alcohol (ethanol) also increases the blood plasma levels of d-methylphenidate by up to 40%.[45]
Liver toxicity from methylphenidate is extremely rare, but limited evidence suggests that intake of β-adrenergic agonists with methylphenidate may increase the risk of liver toxicity.[46]
Mode of activity
Methylphenidate is a catecholamine reuptake inhibitor that indirectly increases catecholaminergic neurotransmission by inhibiting the dopamine transporter (DAT) and norepinephrine transporter (NET),[47] which are responsible for clearing catecholamines from the synapse, particularly in the striatum and meso-limbic system.[48] Moreover, it is thought to "increase the release of these monoamines into the extraneuronal space."[49]
Although four stereoisomers of methylphenidate (MPH) are possible, only the threo diastereoisomers are used in modern practice. There is a high eudysmic ratio between the SS and RR enantiomers of MPH. Dexmethylphenidate (d-threo-methylphenidate) is a preparation of the RR enantiomer of methylphenidate.[50][51] In theory, D-TMP (d-threo-methylphenidate) can be anticipated to be twice the strength of the racemic product.[47][52]
| Compd[53] | DAT (Ki) | DA (IC50) | NET (Ki) | NE (IC50) |
|---|---|---|---|---|
| D-TMP | 161 | 23 | 206 | 39 |
| L-TMP | 2250 | 1600 | >10K | 980 |
| DL-TMP | 121 | 20 | 788 | 51 |
Pharmacology
Dexmethylphenidate has a 4-6 hour duration of effect (a long-acting formulation, Focalin XR, which spans 12 hours is also available and has been shown to be as effective as DL (dextro-, levo-)-TMP (threo-methylphenidate) XR (extended release) (Concerta, Ritalin LA), with flexible dosing and good tolerability.[54][55]) It has also been demonstrated to reduce ADHD symptoms in both children[56] and adults.[57] d-MPH has a similar side-effect profile to MPH[5] and can be administered without regard to food intake.[58]
References
- 1 2 3 4 Moen M, Keam S.Dexmethylphenidate Extended Release: A Review of its Use in the Treatment of Attention-Deficit Hyperactivity Disorder. CNSDrugs 2009; 23(12):1057-1083. doi:10.2165/11201140-000000000-00000.
- ↑ "DAYTRANA" (PDF). United States Food and Drug Administration. Noven Pharmaceuticals, Inc. October 2013. Retrieved 13 June 2014.
- ↑ Methylphenidate Use During Pregnancy and Breastfeeding. Drugs.com. Retrieved on 30 April 2011.
- ↑ Humphreys C, Garcia-Bournissen F, Ito S, Koren G (2007). "Exposure to attention deficit hyperactivity disorder medications during pregnancy". Canadian Family Physician. 53 (7): 1153–5. PMC 1949295
 . PMID 17872810.
. PMID 17872810. - 1 2 Keating, G. M.; Figgitt, D. P. (2002). "Dexmethylphenidate". Drugs. 62 (13): 1899–1904; discussion 1904–8. PMID 12215063. doi:10.2165/00003495-200262130-00009.
- ↑ Didoni, A; Sequi, M; Panei, P; Bonati, M; Lombardy ADHD Registry, Group (October 2011). "One-year prospective follow-up of pharmacological treatment in children with attention-deficit/hyperactivity disorder.". European journal of clinical pharmacology. 67 (10): 1061–7. PMID 21538145. doi:10.1007/s00228-011-1050-3.
• "Ritalin Side Effects". Retrieved 22 June 2015.
• "Biphentin product monograph" (PDF). Purdue Pharma. Archived from the original (PDF) on 22 June 2015. Retrieved 22 June 2015.
• Huss, M; Ginsberg, Y; Tvedten, T; Arngrim, T; Philipsen, A; Carter, K; Chen, CW; Kumar, V (January 2014). "Methylphenidate hydrochloride modified-release in adults with attention deficit hyperactivity disorder: a randomized double-blind placebo-controlled trial.". Advances in therapy. 31 (1): 44–65. PMC 3905180 . PMID 24371021. doi:10.1007/s12325-013-0085-5.
. PMID 24371021. doi:10.1007/s12325-013-0085-5. - 1 2 "Concerta product monograph" (PDF). Janssen Pharmaceuticals. Retrieved 4 December 2016.
- ↑
- ↑ Jaanus SD (1992). "Ocular side-effects of selected systemic drugs". Optom Clin. 2 (4): 73–96. PMID 1363080.
- ↑ Stein; et al. (1998). "Sleep disturbances in children with Attention-Deficit/Hyperactivity Disorder a comparative study with healthy siblings". Journal of Learning Disabilities. 31 (6): 572–578.
- 1 2 Cortese, S; Holtmann, M; Banaschewski, T; Buitelaar, J; Coghill, D; Danckaerts, M; Dittmann, RW; Graham, J; Taylor, E; Sergeant, J; European ADHD Guidelines, Group (March 2013). "Practitioner review: current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents.". Journal of child psychology and psychiatry, and allied disciplines. 54 (3): 227–46. PMID 23294014. doi:10.1111/jcpp.12036.
- ↑ Findling, RL; Dinh, S (March 2014). "Transdermal therapy for attention-deficit hyperactivity disorder with the methylphenidate patch (MTS).". CNS Drugs. 28 (3): 217–28. PMC 3933749
 . PMID 24532028. doi:10.1007/s40263-014-0141-y.
. PMID 24532028. doi:10.1007/s40263-014-0141-y. - ↑ Kraemer M, Uekermann J, Wiltfang J, Kis B (July 2010). "Methylphenidate-induced psychosis in adult attention-deficit/hyperactivity disorder: report of 3 new cases and review of the literature". Clin Neuropharmacol. 33 (4): 204–6. PMID 20571380. doi:10.1097/WNF.0b013e3181e29174.
- ↑ Wingo, AP; Ghaemi, SN (2008). "Frequency of stimulant treatment and of stimulant-associated mania/hypomania in bipolar disorder patients.". Psychopharmacology bulletin. 41 (4): 37–47. PMID 19015628.
- ↑ "Methylphenidate ADHD Medications: Drug Safety Communication – Risk of Long-lasting Erections". U.S. Food and Drug Administration. 17 December 2013. Retrieved 17 December 2013.
- ↑ "FDA Drug Safety Communication: Safety Review Update of Medications used to treat Attention-Deficit/Hyperactivity Disorder (ADHD) in children and young adults". United States Food and Drug Administration. 20 December 2011. Retrieved 4 November 2013.
• Cooper WO, Habel LA, Sox CM, Chan KA, Arbogast PG, Cheetham TC, Murray KT, Quinn VP, Stein CM, Callahan ST, Fireman BH, Fish FA, Kirshner HS, O'Duffy A, Connell FA, Ray WA (November 2011). "ADHD drugs and serious cardiovascular events in children and young adults". N. Engl. J. Med. 365 (20): 1896–1904. PMC 4943074 . PMID 22043968. doi:10.1056/NEJMoa1110212.
. PMID 22043968. doi:10.1056/NEJMoa1110212.
• "FDA Drug Safety Communication: Safety Review Update of Medications used to treat Attention-Deficit/Hyperactivity Disorder (ADHD) in adults". United States Food and Drug Administration. 15 December 2011. Retrieved 4 November 2013.
• Habel LA, Cooper WO, Sox CM, Chan KA, Fireman BH, Arbogast PG, Cheetham TC, Quinn VP, Dublin S, Boudreau DM, Andrade SE, Pawloski PA, Raebel MA, Smith DH, Achacoso N, Uratsu C, Go AS, Sidney S, Nguyen-Huynh MN, Ray WA, Selby JV (December 2011). "ADHD medications and risk of serious cardiovascular events in young and middle-aged adults". JAMA. 306 (24): 2673–2683. PMC 3350308 . PMID 22161946. doi:10.1001/jama.2011.1830.
. PMID 22161946. doi:10.1001/jama.2011.1830. - ↑ Gordon N (1999). "Attention deficit hyperactivity disorder: possible causes and treatment". Int. J. Clin. Pract. 53 (7): 524–8. PMID 10692738.
- 1 2 Noven Pharmaceuticals, Inc. (17 April 2015). "Daytrana Prescribing Information" (PDF). United States Food and Drug Administration. pp. 1–33. Retrieved 23 June 2015.
- 1 2 Heedes G, Ailakis J. "Methylphenidate hydrochloride (PIM 344)". INCHEM. International Programme on Chemical Safety. Retrieved 23 June 2015.
- 1 2 3 Spiller HA, Hays HL, Aleguas A (June 2013). "Overdose of drugs for attention-deficit hyperactivity disorder: clinical presentation, mechanisms of toxicity, and management". CNS Drugs. 27 (7): 531–543. PMID 23757186. doi:10.1007/s40263-013-0084-8.
The management of amphetamine, dextroamphetamine, and methylphenidate overdose is largely supportive, with a focus on interruption of the sympathomimetic syndrome with judicious use of benzodiazepines. In cases where agitation, delirium, and movement disorders are unresponsive to benzodiazepines, second-line therapies include antipsychotics such as ziprasidone or haloperidol, central alpha-adrenoreceptor agonists such as dexmedetomidine, or propofol. ... However, fatalities are rare with appropriate care
- ↑ Bruggisser M, Bodmer M, Liechti ME (2011). "Severe toxicity due to injected but not oral or nasal abuse of methylphenidate tablets". Swiss Med Wkly. 141: w13267. PMID 21984207. doi:10.4414/smw.2011.13267.
- ↑ Nestler EJ, Barrot M, Self DW (September 2001). "DeltaFosB: a sustained molecular switch for addiction". Proc. Natl. Acad. Sci. U.S.A. 98 (20): 11042–11046. PMC 58680
 . PMID 11572966. doi:10.1073/pnas.191352698.
. PMID 11572966. doi:10.1073/pnas.191352698. Although the ΔFosB signal is relatively long-lived, it is not permanent. ΔFosB degrades gradually and can no longer be detected in brain after 1–2 months of drug withdrawal ... Indeed, ΔFosB is the longest-lived adaptation known to occur in adult brain, not only in response to drugs of abuse, but to any other perturbation (that doesn't involve lesions) as well.
- ↑ Nestler EJ (December 2012). "Transcriptional mechanisms of drug addiction". Clin. Psychopharmacol. Neurosci. 10 (3): 136–143. PMC 3569166
 . PMID 23430970. doi:10.9758/cpn.2012.10.3.136.
. PMID 23430970. doi:10.9758/cpn.2012.10.3.136. The 35–37 kD ΔFosB isoforms accumulate with chronic drug exposure due to their extraordinarily long half-lives. ... As a result of its stability, the ΔFosB protein persists in neurons for at least several weeks after cessation of drug exposure. ... ΔFosB overexpression in nucleus accumbens induces NFκB
- ↑ Morton WA, Stockton GG (2000). "Methylphenidate Abuse and Psychiatric Side Effects". Prim Care Companion J Clin Psychiatry. 2 (5): 159–164. PMC 181133
 . PMID 15014637. doi:10.4088/PCC.v02n0502.
. PMID 15014637. doi:10.4088/PCC.v02n0502. - 1 2 3 4 5 Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 15: Reinforcement and Addictive Disorders". In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. p. 368. ISBN 9780071481274.
Cocaine, [amphetamine], and methamphetamine are the major psychostimulants of abuse. The related drug methylphenidate is also abused, although it is far less potent. These drugs elicit similar initial subjective effects ; differences generally reflect the route of administration and other pharmacokinetic factors. Such agents also have important therapeutic uses; cocaine, for example, is used as a local anesthetic (Chapter 2), and amphetamines and methylphenidate are used in low doses to treat attention deficit hyperactivity disorder and in higher doses to treat narcolepsy (Chapter 12). Despite their clinical uses, these drugs are strongly reinforcing, and their long-term use at high doses is linked with potential addiction, especially when they are rapidly administered or when high-potency forms are given.
- 1 2 Steiner H, Van Waes V (January 2013). "Addiction-related gene regulation: risks of exposure to cognitive enhancers vs. other psychostimulants". Prog. Neurobiol. 100: 60–80. PMC 3525776
 . PMID 23085425. doi:10.1016/j.pneurobio.2012.10.001.
. PMID 23085425. doi:10.1016/j.pneurobio.2012.10.001. - ↑ Auger RR, Goodman SH, Silber MH, Krahn LE, Pankratz VS, Slocumb NL (2005). "Risks of high-dose stimulants in the treatment of disorders of excessive somnolence: a case-control study". Sleep. 28 (6): 667–72. PMID 16477952.
- 1 2 3 4 5 6 Kim Y, Teylan MA, Baron M, Sands A, Nairn AC, Greengard P (2009). "Methylphenidate-induced dendritic spine formation and DeltaFosB expression in nucleus accumbens". Proc. Natl. Acad. Sci. U.S.A. 106 (8): 2915–20. PMC 2650365
 . PMID 19202072. doi:10.1073/pnas.0813179106.
. PMID 19202072. doi:10.1073/pnas.0813179106. Despite decades of clinical use of methylphenidate for ADHD, concerns have been raised that long-term treatment of children with this medication may result in subsequent drug abuse and addiction. However, meta analysis of available data suggests that treatment of ADHD with stimulant drugs may have a significant protective effect, reducing the risk for addictive substance use (36, 37). Studies with juvenile rats have also indicated that repeated exposure to methylphenidate does not necessarily lead to enhanced drug-seeking behavior in adulthood (38). However, the recent increase of methylphenidate use as a cognitive enhancer by the general public has again raised concerns because of its potential for abuse and addiction (3, 6–10). Thus, although oral administration of clinical doses of methylphenidate is not associated with euphoria or with abuse problems, nontherapeutic use of high doses or i.v. administration may lead to addiction (39, 40).
- ↑ Elkashef A, Vocci F, Hanson G, White J, Wickes W, Tiihonen J (2008). "Pharmacotherapy of methamphetamine addiction: an update". Substance Abuse. 29 (3): 31–49. PMC 2597382
 . PMID 19042205. doi:10.1080/08897070802218554.
. PMID 19042205. doi:10.1080/08897070802218554. - ↑ Grabowski J, Roache JD, Schmitz JM, Rhoades H, Creson D, Korszun A (1997). "Replacement medication for cocaine dependence: methylphenidate". J Clin Psychopharmacol. 17 (6): 485–8. PMID 9408812. doi:10.1097/00004714-199712000-00008.
- ↑ Gorelick DA, Gardner EL, Xi ZX (2004). "Agents in development for the management of cocaine abuse". Drugs. 64 (14): 1547–73. PMID 15233592. doi:10.2165/00003495-200464140-00004.
- ↑ Karila L, Gorelick D, Weinstein A, Noble F, Benyamina A, Coscas S, Blecha L, Lowenstein W, Martinot JL, Reynaud M, Lépine JP (2008). "New treatments for cocaine dependence: a focused review". Int. J. Neuropsychopharmacol. 11 (3): 425–38. PMID 17927843. doi:10.1017/S1461145707008097.
- ↑ "NIDA InfoFacts: Understanding Drug Abuse and Addiction" (PDF). 2008. Archived from the original (PDF) on 15 December 2010.
- ↑ Shearer J (2008). "The principles of agonist pharmacotherapy for psychostimulant dependence". Drug Alcohol Rev. 27 (3): 301–8. PMID 18368612. doi:10.1080/09595230801927372.
- 1 2 3 Nestler EJ (December 2013). "Cellular basis of memory for addiction". Dialogues Clin. Neurosci. 15 (4): 431–443. PMC 3898681
 . PMID 24459410.
. PMID 24459410. Despite the importance of numerous psychosocial factors, at its core, drug addiction involves a biological process: the ability of repeated exposure to a drug of abuse to induce changes in a vulnerable brain that drive the compulsive seeking and taking of drugs, and loss of control over drug use, that define a state of addiction. ... A large body of literature has demonstrated that such ΔFosB induction in D1-type NAc neurons increases an animal's sensitivity to drug as well as natural rewards and promotes drug self-administration, presumably through a process of positive reinforcement ... Another ΔFosB target is cFos: as ΔFosB accumulates with repeated drug exposure it represses c-Fos and contributes to the molecular switch whereby ΔFosB is selectively induced in the chronic drug-treated state.41. ... Moreover, there is increasing evidence that, despite a range of genetic risks for addiction across the population, exposure to sufficiently high doses of a drug for long periods of time can transform someone who has relatively lower genetic loading into an addict.4
- ↑ Ruffle JK (November 2014). "Molecular neurobiology of addiction: what's all the (Δ)FosB about?". Am J Drug Alcohol Abuse. 40 (6): 428–437. PMID 25083822. doi:10.3109/00952990.2014.933840.
The strong correlation between chronic drug exposure and ΔFosB provides novel opportunities for targeted therapies in addiction (118), and suggests methods to analyze their efficacy (119). Over the past two decades, research has progressed from identifying ΔFosB induction to investigating its subsequent action (38). It is likely that ΔFosB research will now progress into a new era – the use of ΔFosB as a biomarker. ...
Conclusions
ΔFosB is an essential transcription factor implicated in the molecular and behavioral pathways of addiction following repeated drug exposure. The formation of ΔFosB in multiple brain regions, and the molecular pathway leading to the formation of AP-1 complexes is well understood. The establishment of a functional purpose for ΔFosB has allowed further determination as to some of the key aspects of its molecular cascades, involving effectors such as GluR2 (87,88), Cdk5 (93) and NFkB (100). Moreover, many of these molecular changes identified are now directly linked to the structural, physiological and behavioral changes observed following chronic drug exposure (60,95,97,102). New frontiers of research investigating the molecular roles of ΔFosB have been opened by epigenetic studies, and recent advances have illustrated the role of ΔFosB acting on DNA and histones, truly as a ‘‘molecular switch’’ (34). As a consequence of our improved understanding of ΔFosB in addiction, it is possible to evaluate the addictive potential of current medications (119), as well as use it as a biomarker for assessing the efficacy of therapeutic interventions (121,122,124). Some of these proposed interventions have limitations (125) or are in their infancy (75). However, it is hoped that some of these preliminary findings may lead to innovative treatments, which are much needed in addiction.
• Biliński P, Wojtyła A, Kapka-Skrzypczak L, Chwedorowicz R, Cyranka M, Studziński T (2012). "Epigenetic regulation in drug addiction". Ann. Agric. Environ. Med. 19 (3): 491–496. PMID 23020045.For these reasons, ΔFosB is considered a primary and causative transcription factor in creating new neural connections in the reward centre, prefrontal cortex, and other regions of the limbic system. This is reflected in the increased, stable and long-lasting level of sensitivity to cocaine and other drugs, and tendency to relapse even after long periods of abstinence. These newly constructed networks function very efficiently via new pathways as soon as drugs of abuse are further taken ... In this way, the induction of CDK5 gene expression occurs together with suppression of the G9A gene coding for dimethyltransferase acting on the histone H3. A feedback mechanism can be observed in the regulation of these 2 crucial factors that determine the adaptive epigenetic response to cocaine. This depends on ΔFosB inhibiting G9a gene expression, i.e. H3K9me2 synthesis which in turn inhibits transcription factors for ΔFosB. For this reason, the observed hyper-expression of G9a, which ensures high levels of the dimethylated form of histone H3, eliminates the neuronal structural and plasticity effects caused by cocaine by means of this feedback which blocks ΔFosB transcription
• Robison AJ, Nestler EJ (November 2011). "Transcriptional and epigenetic mechanisms of addiction". Nat. Rev. Neurosci. 12 (11): 623–637. PMC 3272277 . PMID 21989194. doi:10.1038/nrn3111.
. PMID 21989194. doi:10.1038/nrn3111. ΔFosB has been linked directly to several addiction-related behaviors ... Importantly, genetic or viral overexpression of ΔJunD, a dominant negative mutant of JunD which antagonizes ΔFosB- and other AP-1-mediated transcriptional activity, in the NAc or OFC blocks these key effects of drug exposure14,22–24. This indicates that ΔFosB is both necessary and sufficient for many of the changes wrought in the brain by chronic drug exposure. ΔFosB is also induced in D1-type NAc MSNs by chronic consumption of several natural rewards, including sucrose, high fat food, sex, wheel running, where it promotes that consumption14,26–30. This implicates ΔFosB in the regulation of natural rewards under normal conditions and perhaps during pathological addictive-like states.
- ↑ Ishii, M; Tatsuzawa, Y; Yoshino, A; Nomura, S (April 2008). "Serotonin syndrome induced by augmentation of SSRI with methylphenidate.". Psychiatry and clinical neurosciences. 62 (2): 246. PMID 18412855. doi:10.1111/j.1440-1819.2008.01767.x.
- ↑ Türkoğlu, S (2015). "Serotonin syndrome with sertraline and methylphenidate in an adolescent.". Clinical neuropharmacology. 38 (2): 65–6. PMID 25768857. doi:10.1097/WNF.0000000000000075.
- ↑ Park, YM; Jung, YK (30 May 2010). "Manic switch and serotonin syndrome induced by augmentation of paroxetine with methylphenidate in a patient with major depression.". Progress in neuro-psychopharmacology & biological psychiatry. 34 (4): 719–20. PMID 20298736. doi:10.1016/j.pnpbp.2010.03.016.
- ↑ Bodner, RA; Lynch, T; Lewis, L; Kahn, D (February 1995). "Serotonin syndrome.". Neurology. 45 (2): 219–23. PMID 7854515. doi:10.1212/wnl.45.2.219.
- ↑ Patrick KS, González MA, Straughn AB, Markowitz JS (2005). "New methylphenidate formulations for the treatment of attention-deficit/hyperactivity disorder". Expert Opinion on Drug Delivery. 2 (1): 121–43. PMID 16296740. doi:10.1517/17425247.2.1.121.
- ↑ Markowitz JS, DeVane CL, Boulton DW, Nahas Z, Risch SC, Diamond F, Patrick KS (2000). "Ethylphenidate formation in human subjects after the administration of a single dose of methylphenidate and ethanol". Drug Metabolism and Disposition. 28 (6): 620–4. PMID 10820132.
- ↑ Markowitz JS, Logan BK, Diamond F, Patrick KS (1999). "Detection of the novel metabolite ethylphenidate after methylphenidate overdose with alcohol coingestion". Journal of Clinical Psychopharmacology. 19 (4): 362–6. PMID 10440465. doi:10.1097/00004714-199908000-00013.
- ↑ Markowitz JS, DeVane CL, Boulton DW, Nahas Z, Risch SC, Diamond F, Patrick KS (2000). "Ethylphenidate formation in human subjects after the administration of a single dose of methylphenidate and ethanol". Drug metabolism and disposition: the biological fate of chemicals. 28 (6): 620–4. PMID 10820132.
- ↑ Patrick KS, Straughn AB, Minhinnett RR, Yeatts SD, Herrin AE, DeVane CL, Malcolm R, Janis GC, Markowitz JS (March 2007). "Influence of ethanol and gender on methylphenidate pharmacokinetics and pharmacodynamics.". Clinical pharmacology and therapeutics. 81 (3): 346–53. PMC 3188424
 . PMID 17339864. doi:10.1038/sj.clpt.6100082.
. PMID 17339864. doi:10.1038/sj.clpt.6100082. - ↑ Roberts SM, DeMott RP, James RC (1997). "Adrenergic modulation of hepatotoxicity". Drug Metab. Rev. 29 (1–2): 329–53. PMID 9187524. doi:10.3109/03602539709037587.
- 1 2 Markowitz, John S.; Patrick, Kennerly S. (2008). "Differential Pharmacokinetics and Pharmacodynamics of Methylphenidate Enantiomers". Journal of Clinical Psychopharmacology. 28 (Suppl. 2): S54–S61. ISSN 0271-0749. PMID 18480678. doi:10.1097/JCP.0b013e3181733560.
- ↑ Schweri, M. M.; Skolnick, P.; Rafferty, M. F.; Rice, K. C.; Janowsky, A. J.; Paul, S. M. (1985). "3HThreo-(+/-)-methylphenidate binding to 3,4-dihydroxyphenylethylamine uptake sites in corpus striatum: correlation with the stimulant properties of ritalinic acid esters". Journal of Neurochemistry. 45 (4): 1062–1070. PMID 4031878. doi:10.1111/j.1471-4159.1985.tb05524.x.
- ↑ Novartis: FOCALIN XR Overview PDF: FOCALIN XR - Full Prescribing Information
- ↑ Ding, Y. S.; Fowler, J. S.; Volkow, N. D.; Dewey, S. L.; Wang, G. J.; Logan, J.; Gatley, S. J.; Pappas, N. (1997). "Chiral drugs: comparison of the pharmacokinetics of 11Cd-threo and L-threo-methylphenidate in the human and baboon brain". Psychopharmacology. 131 (1): 71–78. PMID 9181638. doi:10.1007/s002130050267.
- ↑ Ding, Y.; Gatley, S.; Thanos, P.; Shea, C.; Garza, V.; Xu, Y.; Carter, P.; King, P.; Warner, D.; Taintor, N. B.; Park, D. J.; Pyatt, B.; Fowler, J. S.; Volkow, N. D. (2004). "Brain kinetics of methylphenidate (Ritalin) enantiomers after oral administration". Synapse. 53 (3): 168–175. PMID 15236349. doi:10.1002/syn.20046.
- ↑ Davids, E.; Zhang, K.; Tarazi, F.; Baldessarini, R. (2002). "Stereoselective effects of methylphenidate on motor hyperactivity in juvenile rats induced by neonatal 6-hydroxydopamine lesioning". Psychopharmacology. 160 (1): 92–98. PMID 11862378. doi:10.1007/s00213-001-0962-5.
- ↑ Williard, R.; Middaugh, L.; Zhu, H.; Patrick, K. (2007). "Methylphenidate and its ethanol transesterification metabolite ethylphenidate: brain disposition, monoamine transporters and motor activity". Behavioural Pharmacology. 18 (1): 39–51. PMID 17218796. doi:10.1097/FBP.0b013e3280143226.
- ↑ McGough, J.; Pataki, C. S.; Suddath, R. (2005). "Dexmethylphenidate extended-release capsules for attention deficit hyperactivity disorder". Expert Review of Neurotherapeutics. 5 (4): 437–441. PMID 16026226. doi:10.1586/14737175.5.4.437.
- ↑ Silva, R.; Tilker, H. A.; Cecil, J. T.; Kowalik, S.; Khetani, V.; Faleck, H.; Patin, J. (2004). "Open-label study of dexmethylphenidate hydrochloride in children and adolescents with attention deficit hyperactivity disorder". Journal of child and adolescent psychopharmacology. 14 (4): 555–563. PMID 15662147. doi:10.1089/cap.2004.14.555.
- ↑ Arnold, L.E.; et al. (Winter 2004). "A double-blind, placebo-controlled withdrawal trial of dexmethylphenidate hydrochloride in children with attention deficit hyperactivity disorder". J Child Adolesc Psychopharmacol. 14 (4): 542–54. doi:10.1089/cap.2004.14.542.
- ↑ Spencer, T.; Adler, L.; Mcgough, J.; Muniz, R.; Jiang, H.; Pestreich, L.; Adult Adhd Research, G. (2007). "Efficacy and safety of dexmethylphenidate extended-release capsules in adults with attention-deficit/hyperactivity disorder". Biological Psychiatry. 61 (12): 1380–1387. PMID 17137560. doi:10.1016/j.biopsych.2006.07.032.
- ↑ Teo, S. K.; Scheffler, M. R.; Wu, A.; Stirling, D. I.; Thomas, S. D.; Stypinski, D.; Khetani, V. D. (2004). "A single-dose, two-way crossover, bioequivalence study of dexmethylphenidate HCl with and without food in healthy subjects". Journal of clinical pharmacology. 44 (2): 173–178. PMID 14747426. doi:10.1177/0091270003261899.