Cyclononatetraene
 | |
| Identifiers | |
|---|---|
| 3D model (JSmol) |
|
| ChemSpider | |
| |
| |
| Properties | |
| C9H10 | |
| Molar mass | 118.18 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
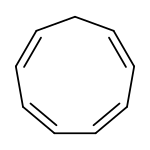
Cyclononatetraene is a organic compound with the formula C9H10. It is unstable and reorganized to cis-8,9-dihydroindene.[1]
Cyclononatetraenyl anion
Cyclononatetraenyl anion is stable. There are two cyclononatetraenyl anion:trans,cis,cis,cis-Cyclononatetraenyl Anion(Pac-Man shape) and cis,cis,cis,cis-cyclononatetraenyl anion(regular nonagon shape). cis,cis,cis,cis-cyclononatetraenyl anion is more stable than trans,cis,cis,cis-cyclononatetraenyl anion.[2]
Cyclononatetraenyl cation
Cyclononatetraenyl cation is also stable by Möbius aromaticity.[3][4]
See also
References
- ↑ Radlick, Phillip; Gary Alford (1969), "Preparation and isolation of cis, cis, cis, cis-1,3,5,7-cyclononatetraene", J. Am. Chem. Soc, 91 (23): 6529–6530, doi:10.1021/ja01051a083
- ↑ Radlick, Phillip; Gary Alford (1969), "trans,cis,cis,cis-Cyclononatetraenyl Anion, a New Aromatic 10 π System", Angewandte Chemie International Edition in English, 8 (12): 984, doi:10.1002/anie.196909841
- ↑ Thermal bicyclo[6.1.0]nonatrienyl chloride-dihydroindenyl chloride rearrangement Paul v. R. Schleyer, James C. Barborak, Tah Mun Su, Gernot Boche, and G. Schneider J. Am. Chem. Soc.; 1971; 93(1) pp 279 - 281; doi:10.1021/ja00730a063
- ↑ Topology in Chemistry: Designing Möbius Molecules Herges, R. Chem. Rev.; (Review); 2006; 106(12); 4820-4842. doi:10.1021/cr0505425
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.