Cryptosporidiosis
| Cryptosporidiosis | |
|---|---|
 | |
| Micrograph showing cryptosporidiosis. The cryptosporidium are the small, round bodies in apical vacuoles on the surface of the epithelium. H&E stain. Colonic biopsy. | |
| Classification and external resources | |
| Specialty | infectious disease |
| ICD-10 | A07.2 |
| ICD-9-CM | 007.4 |
| DiseasesDB | 3221 |
| MedlinePlus | 000617 |
| eMedicine | med/484 |
| Patient UK | Cryptosporidiosis |
Cryptosporidiosis, also known as crypto,[1] is a parasitic disease caused by Cryptosporidium, a genus of protozoan parasites in the phylum Apicomplexa. It affects the distal small intestine and can affect the respiratory tract in both immunocompetent (i.e., individuals with a normal functioning immune system) and immunocompromised (e.g., persons with HIV/AIDS or autoimmune disorders) individuals, resulting in watery diarrhea with or without an unexplained cough.[2] In immunocompromised individuals, the symptoms are particularly severe and can be fatal. It is primarily spread through the fecal-oral route, often through contaminated water;[2][3] recent evidence suggests that it can also be transmitted via fomites in respiratory secretions.[2]
Cryptosporidium is the organism most commonly isolated in HIV-positive patients presenting with diarrhea. Despite not being identified until 1976, it is one of the most common waterborne diseases and is found worldwide. The parasite is transmitted by environmentally hardy microbial cysts (oocysts) that, once ingested, sporozoites within oocysts excyst (i.e., are released) and result in an infection of intestinal epithelial tissue.
Signs and symptoms
Cryptosporidiosis may occur as an asymptomatic infection, an acute infection (i.e., duration shorter than 2 weeks), as recurrent acute infections in which symptoms reappear following a brief period of recovery for up to 30 days, and as a chronic infection (i.e., duration longer than 2 weeks) in which symptoms are severe and persistent.[2][4][5][6] It may be fatal in individuals with a severely compromised immune system.[2][4] Symptoms usually appear 5–10 days after infection (range: 2–28 days) and normally last for up to 2 weeks in immunocompetent individuals;[2][4][5] symptoms are usually more severe and persist longer in immunocompromised individuals.[2][4][5] Following the resolution of diarrhea, symptoms can reoccur after several days or weeks due to reinfection.[4][5][6][7] Based upon one clinical trial, the likelihood of re-infection is high in immunocompetent adults.[7]
In immunocompetent individuals, cryptosporidiosis is primarily localized to the distal small intestine and sometimes the respiratory tract as well.[2][5] In immunocompromised persons, cryptosporidiosis may disseminate to other organs, including the hepatobiliary system, pancreas, upper gastrointestinal tract, and urinary bladder;[2][5] pancreatic and biliary infection can involve acalculous cholecystitis, sclerosing cholangitis, papillary stenosis, or pancreatitis.[5][8]
Intestinal cryptosporidiosis
Common signs and symptoms of intestinal cryptosporidiosis include:
- Moderate to severe watery diarrhea,[2][4][5] sometimes contains mucus and rarely contains blood or leukocytes[5]
- In very severe cases, diarrhea may be profuse and cholera-like with malabsorption and hypovolemia[5]
- Low-grade fever[2][4][5]
- Crampy abdominal pain[2][4][5]
- Dehydration[2][4]
- Weight loss[2][4]
- Fatigue[7]
- Nausea and vomiting[2][4][5] – suggests upper GI tract involvement[5] and may lead to respiratory cryptosporidiosis[2]
- Epigastric or right upper quadrant tenderness[5]
Less common or rare signs and symptoms include:
- Reactive arthritis (may affect the hands, knees, ankles, and feet)[5]
- Jaundice – suggests hepatobiliary involvement[5]
- Ascites – suggests pancreatic involvement[5]
Respiratory cryptosporidiosis
Symptoms of upper respiratory cryptosporidiosis include:
- Inflammation of the nasal mucosa, sinuses, larynx, or trachea[2]
- Nasal discharge[2]
- Voice change[2] (e.g., hoarseness)[5]
Symptoms of lower respiratory cryptosporidiosis include:
Cause
Cryptosporidium is a genus of protozoan pathogens which is categorized under the phylum Apicomplexa. Other apicomplexan pathogens include the malaria parasite Plasmodium, and Toxoplasma, the causative agent of toxoplasmosis. A number of Cryptosporidium infect mammals. In humans, the main causes of disease are C. parvum and C. hominis (previously C. parvum genotype 1). C. canis, C. felis, C. meleagridis, and C. muris can also cause disease in humans. Cryptosporidium is capable of completing its life cycle within a single host, resulting in microbial cyst stages that are excreted in feces and are capable of transmission to a new host via the fecal-oral route. Other vectors of disease transmission also exist.[2][9]
The pattern of Cryptosporidium life cycle fits well with that of other intestinal homogeneous coccidian genera of the suborder Eimeriina: macro- and microgamonts develop independently; a microgamont gives rise to numerous male gametes; and oocysts serving for parasites' spreading in the environment. Electron microscopic studies made from the 1970s have shown the intracellular, although extracytoplasmic localization of Cryptosporidium species.
These species possess a number of unusual features:
- an endogenous phase of development in microvilli of epithelial surfaces
- two morphofunctional types of oocysts
- the smallest number of sporozoites per oocyst
- a multi-membraneous "feeder" organelle
DNA studies suggest a relationship with the gregarines rather than the coccidia.[10] The taxonomic position of this group has not yet been finally agreed upon.
The genome of Cryptosporidium parvum was sequenced in 2004 and was found to be unusual amongst Eukaryotes in that the mitochondria seem not to contain DNA.[11] A closely related species, C. hominis, also has its genome sequence available.[12] CryptoDB.org is a NIH-funded database that provides access to the Cryptosporidium genomics data sets.
Transmission
Infection is through contaminated material such as earth, water, uncooked or cross-contaminated food that has been in contact with the feces of an infected individual or animal. Contact must then be transferred to the mouth and swallowed. It is especially prevalent amongst those in regular contact with bodies of fresh water including recreational water such as swimming pools. Other potential sources include insufficiently treated water supplies, contaminated food, or exposure to feces.[3] The high resistance of Cryptosporidium oocysts to disinfectants such as chlorine bleach enables them to survive for long periods and still remain infective.[13] Some outbreaks have happened in day care related to diaper changes.[14]
The following groups have an elevated risk of being exposed to Cryptosporidium:[3]
- Child care workers
- Parents of infected children
- People who take care of other people with cryptosporidiosis
- International travelers
- Backpackers, hikers, and campers who drink unfiltered, untreated water
- People, including swimmers, who swallow water from contaminated sources
- People who handle infected cattle
- People exposed to human feces through sexual contact
Cases of cryptosporidiosis can occur in a city that does not have a contaminated water supply. In a city with clean water, it may be that cases of cryptosporidiosis have different origins. Testing of water, as well as epidemiological study, are necessary to determine the sources of specific infections. Cryptosporidium is causing serious illness [15] more frequently in immunocompromised than in apparently healthy individuals. It may chronically sicken some children, as well as adults who are exposed and immunocompromised. A subset of the immunocompromised population is people with AIDS. Some sexual behaviors can transmit the parasite directly.[3]
Life cycle
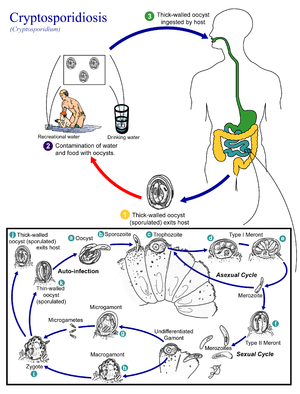
Cryptosporidium spp. exist as multiple cell types which correspond to different stages in an infection (e.g., a sexual and asexual stage).[1] As an oocyst – a type of hardy, thick-walled spore – it can survive in the environment for months and is resistant to many common disinfectants, particularly chlorine-based disinfectants.[16][17] After being ingested, the sporozoites within oocysts excyst (i.e., are released) in the small intestine. The released sporozoites subsequently attach to the microvilli of the epithelial cells of the small intestine. From there they become trophozoites that reproduce asexually by multiple fission, a process known as schizogony. The trophozoites develop into Type 1 meronts [1] that contain 8 daughter cells.[18]
These daughter cells are Type 1 merozoites, which get released by the meronts. Some of these merozoites can cause autoinfection by attaching to epithelial cells. Others of these merozoites become Type II meronts,[19] which contain 4 Type II merozoites.[18] These merozoites get released and they attach to the epithelial cells. From there they become either macrogamonts or microgamonts.[19] These are the female and male sexual forms, respectively.[18] This stage, when sexual forms arise, is called gametogony.[20]
Zygotes are formed by microgametes from the microgamont penetrating the macrogamonts. The zygotes develop into oocysts of two types.[19] 20% of oocysts have thin walls and so can reinfect the host by rupturing and releasing sporozoites that start the process over again.[18] The thick-walled oocysts are excreted into the environment.[19] The oocysts are mature and infective upon being excreted.[18]
Pathogenesis
The oocysts are ovoid or spherical and measure 5 to 6 micrometers across. When in flotation preparations they appear highly refractile. The oocysts contains up to 4 sporozoites that are bow-shaped.[21]
As few as 2 to 10 oocysts can initiate an infection.[22] The parasite is located in the brush border of the epithelial cells of the small intestine.[23] They are mainly located in the jejunum. When the sporozoites attach the epithelial cells’ membrane envelops them. Thus, they are “intracellular but extracytoplasmic”.[18] The parasite can cause damage to the microvilli where it attaches.[21] The infected human excretes the most oocysts during the first week.[18] Oocysts can be excreted for weeks after the diarrhea subsides from infections by C. parvum or C. hominis;[1] however, immunocompetent individuals with C. muris infections have been observed excreting oocysts for seven months.[24]
The immune system reduces the formation of Type 1 merozoites as well as the number of thin-walled oocysts.[18] This helps prevent autoinfection. B cells do not help with the initial response or the fight to eliminate the parasite.[22] Previous infection in immunocompetent individuals produces little resistance to future infection, however it may decrease the severity of disease and the number of oocysts excreted.[25][26]
Diagnosis
There are many diagnostic tests for Cryptosporidium. They include microscopy, staining, and detection of antibodies. Microscopy[1] can help identify oocysts in fecal matter.[23] To increase the chance of finding the oocysts, the diagnostician should inspect at least 3 stool samples.[20] There are several techniques to concentrate either the stool sample or the oocysts. The modified formalin-ethyl acetate (FEA) concentration method concentrates the stool.[21] Both the modified zinc sulfate centrifugal flotation technique and the Sheather’s sugar flotation procedure can concentrate the oocysts by causing them to float.[20] Another form of microscopy is fluorescent microscopy done by staining with auramine.[23]
Other staining techniques include acid-fast staining,[22] which will stain the oocysts red.[21] One type of acid-fast stain is the Kinyoun stain.[17] Giemsa staining can also be performed.[18] Part of the small intestine can be stained with hematoxylin and eosin (H & E), which will show oocysts attached to the epithelial cells.[21]
Detecting antigens is yet another way to diagnose the disease. This can be done with direct fluorescent antibody (DFA) techniques.[1] It can also be achieved through indirect immunofluorescence assay.[20] Enzyme-linked immunosorbent assay (ELISA) also detects antigens.[23]
Polymerase chain reaction (PCR) is another way to diagnose cryptosporidiosis. It can even identify the specific species of Cryptosporidium.[1] If the patient is thought to have biliary cryptosporidiosis, then an appropriate diagnostic technique is ultrasonography. If that returns normal results, the next step would be to perform endoscopic retrograde cholangiopancreatography.[22]
Prevention
Many treatment plants that take raw water from rivers, lakes, and reservoirs for public drinking water production use conventional filtration technologies. This involves a series of processes, including coagulation, flocculation, sedimentation, and filtration. Direct filtration, which is typically used to treat water with low particulate levels, includes coagulation and filtration, but not sedimentation. Other common filtration processes, including slow sand filters, diatomaceous earth filters and membranes will remove 99% of Cryptosporidium.[27] Membranes and bag and cartridge filters remove Cryptosporidium product-specifically.
While Cryptosporidium is highly resistant to chlorine disinfection,[28] with high enough concentrations and contact time, Cryptosporidium will be inactivated by chlorine dioxide and ozone treatment. The required levels of chlorine generally preclude the use of chlorine disinfection as a reliable method to control Cryptosporidium in drinking water. Ultraviolet light treatment at relatively low doses will inactivate Cryptosporidium. Water Research Foundation-funded research originally discovered UV's efficacy in inactivating Cryptosporidium.[29][30]
One of the largest challenges in identifying outbreaks is the ability to identify Cryptosporidium in the laboratory. Real-time monitoring technology is now able to detect Cryptosporidium with online systems, unlike the spot and batch testing methods used in the past.
The most reliable way to decontaminate drinking water that may be contaminated by Cryptosporidium is to boil it.[31][32]
In the US the law requires doctors and labs to report cases of cryptosporidiosis to local or state health departments. These departments then report to the Center for Disease Control and Prevention.[1] The best way to prevent getting and spreading cryptosporidiosis is to have good hygiene and sanitation.[20] An example would be hand-washing.[1] Prevention is through washing hands carefully after going to the bathroom or contacting stool, and before eating. People should avoid contact with animal feces.[23] They should also avoid possibly contaminated food and water.[1] In addition, people should refrain from engaging in sexual activities that can expose them to feces.[20]
Standard water filtration may not be enough to eliminate Cryptosporidium; boiling for at least 1 minute (3 minutes above 6,500 feet (2,000 m) of altitude) will decontaminate it. Heating milk at 71.7 °C (161 °F) for 15 seconds pasteurizes it and can destroy the oocysts' ability to infect.[33] Water can also be made safe by filtering with a filter with pore size not greater than 1 micrometre, or by filters that have been approved for “cyst removal” by NSF International National Sanitation Foundation.[1] Bottled drinking water is less likely to contain Cryptosporidium, especially if the water is from an underground source.[33]
People with cryptosporidiosis should not swim in communal areas because the pathogen can reside in the anal and genital areas and be washed off. They should wait until at least two weeks after diarrhea stops before entering public water sources, since oocysts can still be shed for a while. Also, they should stay away from immunosuppressed people.[1] Immunocompromised people should take care to protect themselves from water in lakes and streams.[22] They should also stay away from animal stools and wash their hands after touching animals. To be safe, they should boil or filter their water. They should also wash and cook their vegetables.[1]
The US CDC notes the recommendation of many public health departments to soak contaminated surfaces for 20 minutes with a 3% hydrogen peroxide (99% kill rate) and then rinse them thoroughly, with the caveat that no disinfectant is guaranteed to be completely effective against Cryptosporidium. However, hydrogen peroxide is more effective than standard bleach solutions.[34]
Treatment
Symptomatic treatment primarily involves fluid rehydration, electrolyte replacement (sodium, potassium, bicarbonate, and glucose), and antimotility agents (e.g., loperamide).[35][36] Supplemental zinc may improve symptoms,[35] particularly in recurrent or persistent infections or in others at risk for zinc deficiency.
Immunocompetent
Immunocompetent individuals with cryptosporidiosis typically suffer a short (i.e., duration of less than 2 weeks) self-limiting course of diarrhea that may require symptomatic treatment and ends with spontaneous recovery; in some circumstances, antiparasitic medication may be required (e.g., recurrent, severe, or persistent symptoms);[7] however reinfection frequently occurs.[7]
As of 2015, nitazoxanide is the only antiparasitic drug treatment with proven efficacy for cryptosporidiosis in immunocompetent individuals;[7][35][36][37] however, it lacks efficacy in severely immunocompromised patients.[37] Certain agents such as paromomycin and azithromycin are sometimes used as well, but they only have partial efficacy.[35]
Immunocompromised
In immunocompromised individuals, such as AIDS patients, cryptosporidiosis resolves slowly or not at all, and frequently causes a particularly severe and persistent form of watery diarrhea coupled with a greatly decreased ability to absorb key nutrients through the intestinal tract. As a result, infected individuals may experience severe dehydration, electrolyte imbalances, malnutrition, wasting, and potentially death. In general, the mortality rate for infected AIDS patients is based on CD4+ marker counts. Patients with CD4+ counts over 180 cells/mm³ recover with supportive hospital care and medication; but, in patients with CD4+ counts below 50 cells/mm³, the effects are usually fatal within 3 to 6 months. During the Milwaukee cryptosporidiosis epidemic (the largest of its kind), 73% of AIDS patients with CD4+ counts lower than 50 cells/mm³ and 36% of those with counts between 50 and 200 cells/mm³ died within the first year of contracting the infection.[38]
The best treatment approach is to improve the immune status in immunodeficient individuals using highly active antiretroviral therapy that includes an HIV protease inhibitor along with continued use of antiparasitic medication.[35][36] Antiparasitic drug treatment for immunocompromised individuals usually involves the combination of nitazoxanide, paromomycin, and azithromycin together;[7][35] these drugs are only partially active in HIV/AIDS patients compared to their effect in immunocompetent persons.[35] A Cochrane Collaboration review recommended that nitazoxanide be considered for use in treatment despite its reduced effectiveness in immunocompromised individuals.[36]
Currently, research is being done in molecular-based immunotherapy. For example, synthetic isoflavone derivates have been shown to fight off Cryptosporidium parvum both in vitro and in animal studies. Derivates of nitazoxanide, known as thiazolides, have also shown promising results in vitro.[39]
Epidemiology
Cryptosporidiosis is found worldwide. It causes 50.8% of water-borne diseases that are attributed to parasites.[17] In developing countries, 8–19% of diarrheal diseases can be attributed to Cryptosporidium.[40] Ten percent of the population in developing countries excretes oocysts. In developed countries, the number is lower at 1–3%. The age group most affected are children from 1 to 9 years old.[22][41]
Roughly 30% of adults in the United States are seropositive for cryptosporidiosis, meaning that they contracted the infection at some point in their lives.[7]
History
The organism was first described in 1907 by Tyzzer, who recognised it was a coccidian.[42]
Research
A recombinant Cryptosporidium parvum oocyst surface protein (rCP15/60) vaccine has produced an antibody response in a large group of cows and also antibody response in calves fed rCP15/60-immune colostrum produced by these vaccinated cows. This is very promising. Human Cryptosporidium parvum infections are particularly prevalent and often fatal in neonates in developing countries and to immunocompromised people, such as AIDS patients. There is no commercially available effective vaccine against Cryptosporidium parvum, although passive immunization utilizing different zoite surface (glyco)proteins has shown promise. Developmental stages of the life cycle of the parasite might act as possible targets for vaccine development. The organism is detected in 65–97% of the surface-water supply in the United States and is resistant to most disinfectants used for the treatment of drinking water. Antibodies in the serum of humans and animals infected with Cryptosporidium parvum react with several antigens, one of which is a 15 kDa protein (CP15) located on the surface of the organism. This protein is a good candidate for use as a molecular vaccine because previous studies have shown that a monoclonal antibody to CP15 confers passive immunity to mice. Currently, there is no vaccine or completely effective drug therapy against Cryptosporidium parvum in HIV/AIDS individuals.[35][36]
Other animals
The most important zoonotic reservoirs are cattle,[43] sheep and goats. In addition, in recent years, cryptosporidiosis has plagued many commercial leopard gecko breeders. Several species of the Cryptosporidium family (C. serpentes and others) are involved, and outside of geckos it has been found in monitor lizards, iguanas and tortoises, as well as several snake species.
Notable cases
Before 2000
- In 1987, 13,000 people in Carrollton, Georgia, United States, became ill with cryptosporidiosis. This was the first report of its spread through a municipal water system that met all state and federal drinking water standards.
- In 1993, a waterborne cryptosporidiosis outbreak occurred in Milwaukee, Wisconsin, US. An estimated 403,000 people became ill, including 4,400 people hospitalized. An estimated 69 people died during the outbreak, according to the CDC.[44]
- The UK's biggest outbreak occurred in Torbay in Devon in 1995.
- In the summer of 1996, Cryptosporidium affected approximately 2,000 people in Cranbrook, British Columbia, Canada. Weeks later, a separate incident occurred in Kelowna, British Columbia, where 10,000 to 15,000 people got sick.[45]
2001–2009
- In April 2001, an outbreak occurred in the city of North Battleford, Saskatchewan, Canada. Between 5800 and 7100 people suffered from diarrheal illness, and 1907 cases of cryptosporidiosis were confirmed. Equipment failures at the city's antiquated water filtration plant following maintenance were found to have caused the outbreak.[46]
- In the summer of 2005, after numerous reports by patrons of gastrointestinal upset, a water park at Seneca Lake State Park, in the Finger Lakes region of upstate New York was found to have two water storage tanks infected with Cryptosporidium. By early September 2005, over 3,800 people reported symptoms of a Cryptosporidium infection.[47] The "Sprayground" was ordered closed for the season on 15 August.
- In October 2005, the Gwynedd and Anglesey areas of North Wales, the United Kingdom, suffered an outbreak of cryptosporidiosis. The outbreak may have been linked to the drinking water supply from Llyn Cwellyn, but this is not yet confirmed. As a result, 231 people fell ill and the company Welsh Water (Dwr Cymru) advised 61,000 people to boil their water before use.
- In March 2007, a suspected outbreak occurred in Galway, Ireland, after the source of water for much of the county, Lough Corrib, was suspected to be contaminated with the parasite. A large population (90,000 people), including areas of both Galway City and County, were advised to boil water for drinking, food preparation and for brushing teeth. On 21 March 2007, it was confirmed that the city and county's water supply was contaminated with the parasite. The area's water supply was finally given approval on 20 August 2007, five months after Cryptosporidium was first detected. Around 240 people are known to have contracted the disease; experts say the true figure could be up to 5,000.[48]
- Hundreds of public pools in 20 Utah counties were closed to young children in 2007, as children under 5 are most likely to spread the disease, especially children wearing diapers. As of 10 September 2007 the Utah Department of Health had reported 1302 cases of cryptosporidiosis in the year; a more usual number would be 30. On 25 September the pools were reopened to those not requiring diapers, but hyperchlorination requirements were not lifted.
- On 21 September 2007, a Cryptosporidium outbreak attacked the Western United States: 230 Idaho residents, with hundreds across the Rocky Mountain area; in the Boise and Meridian areas; Utah, 1,600 illnesses; Colorado and other Western states — Montana, decrease.[49]
- On 25 June 2008, Cryptosporidium was found in England in water supplies in Northampton, Daventry, and some surrounding areas supplied from the Pitsford Reservoir, as reported on the BBC. People in the affected areas were warned not to drink tap water unless it had been boiled. Anglian Water confirmed that 108,000 households were affected, about 250,000 people. They advised that water might not be fit for human consumption for many weeks.[50] The boil notice was lifted for all the affected customers on 4 July 2008.[51]
- Throughout the summer of 2008; many public swimming areas, water parks, and public pools in the Dallas/Fort Worth Metroplex of Texas suffered an outbreak of cryptosporidiosis. Burger's Lake in Fort Worth was the first to report such an outbreak. This prompted some, if not all, city-owned and private pools to close and hyperchlorinate. To the 13 August 2008 there were 400 reported cases of Cryptosporidium.[52]
- In September 2008, a gym in Cambridge, the United Kingdom, was forced to close its swimming pool until further notice after health inspectors found an outbreak of cryptosporidiosis. Environmental Health authorities requested that the water be tested after it was confirmed that a young man had been infected.[53]
2010 and later
- In May 2010, the Behana creek water supply south of Cairns, Australia, was found to be contaminated by cryptosporidium.[54]
- In July 2010, a local sports center in Cumbernauld (Glasgow, UK) detected traces of cryptosporidium in its swimming pools, causing a temporary closure of the swimming pools.
- In November 2010, over 4000 cases of cryptosporidiosis were reported in Östersund, Sweden. The source of contamination was the tap water.[55] In mid December 2010 the number of reported cases was 12,400 according to local media.[56]
- As of April 2011, there has been an ongoing outbreak in Skellefteå, Sweden. Although many people have been diagnosed with cryptosporidiosis, the source of the parasite has not yet been found. Several tests have been taken around the water treatment unit "Abborren", but so far no results have turned up positive. Residents are being advised to boil the tap water as they continue to search for the contaminating source.
- Since May 2011, there has been an ongoing outbreak in South Roscommon in Ireland. Although many people have been diagnosed with cryptosporidiosis, the source of the parasite has not yet been found. Testing continues and Roscommon County Council are now considering introducing Ultra Violet Filtration to their water treatment process in the next 12 months. Residents are being advised to boil the tap water and there is no sign of this boil notice being lifted in the near future.
- In May 2013, in Roscommon, Ireland, another outbreak of the cryptosporidiosis was reported and a boil water notice was issued. This was the second time the parasite was detected in a month in the Roscommon water supply. The source of one of the outbreaks had been linked to the agricultural community.[57] To date, 13 people have been treated for Cryptosporidiosis and the boil water notice is still in effect.[58]
See also
- Cryptosporidium was the basis of the 1998 television film, Thirst,[59] in which it mutates and passes through a town's water filters.
- Cryptosporidium was shown on three episodes in three seasons of the television show, Monsters Inside Me
References
- 1 2 3 4 5 6 7 8 9 10 11 12 "Cryptosporidiosis". Centers for Disease Control and Prevention. 5 February 2009.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 Sponseller JK, Griffiths JK, Tzipori S (2014). "The evolution of respiratory Cryptosporidiosis: evidence for transmission by inhalation". Clin. Microbiol. Rev. 27 (3): 575–86. PMC 4135895
 . PMID 24982322. doi:10.1128/CMR.00115-13.
. PMID 24982322. doi:10.1128/CMR.00115-13. Recent evidence indicates that respiratory cryptosporidiosis may occur commonly in immunocompetent children with cryptosporidial diarrhea and unexplained cough. Findings from animal models, human case reports, and a few epidemiological studies suggest that Cryptosporidium may be transmitted via respiratory secretions, in addition to the more recognized fecal-oral route. ... Upper respiratory cryptosporidiosis may cause inflammation of the nasal mucosa, sinuses, larynx, and trachea, accompanied by nasal discharge and voice change (54, 61, 62). Cryptosporidiosis of the lower respiratory tract typically results in productive cough, dyspnea, fever, and hypoxemia (63,–66). ... While fecal-oral transmission is indisputably the major route of infection, transmission via coughing and fomites is also possible in situations of close contact (20). ... Because they lacked gastrointestinal symptoms and oocyst excretion, the latter cases establish the possibility of primary respiratory infection with Cryptosporidium, which may have been acquired by inhalation of expectorated droplets or by contact with fomites. ... This finding suggests that respiratory cryptosporidiosis may occur commonly in immunocompetent individuals.
- 1 2 3 4 "Cryptosporidium: Sources of Infection & Risk Factors". United States Centers for Disease Control and Prevention. 1 April 2015. Retrieved 16 January 2016.
- 1 2 3 4 5 6 7 8 9 10 11 "Cryptosporidium: Illness & Symptoms". United States Centers for Disease Control and Prevention. 20 February 2015. Retrieved 11 January 2016.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 Cabada MM, White AC, Venugopalan P, Sureshbabu J (18 August 2015). Bronze MS, ed. "Cryptosporidiosis Clinical Presentation". Medscape. WebMD. Retrieved 8 January 2016.
After an incubation period of 5–10 days (range 2–28 days), an infected individual develops watery diarrhea ... fever may be low grade or nonexistent; ... Diarrhea, with or without crampy abdominal pain, may be intermittent and scant or continuous, watery, and copious; sometimes, the diarrhea is mucoid. ... Biliary tract involvement is seen in persons with AIDS who have very low CD4 cell counts and is common in children with X-linked immunodeficiency with hyper–immunoglobulin M (IgM). ... Other signs related to GI illness include right upper-quadrant or epigastric tenderness, icterus, and, rarely, ascites related to pancreatic involvement. Reactive arthritis that affects the hands, knees, ankles, and feet has been described.
- 1 2 "Cryptosporidium: Nitazoxanide". United States Centers for Disease Control and Prevention. 20 February 2015. Retrieved 11 January 2016.
Healthcare professionals might consider re-testing stool at least 1 week after the last dose of nitazoxanide only if symptoms do not resolve. In such cases, longer courses of treatment might be needed. Persistent symptoms may also represent re-infection
- 1 2 3 4 5 6 7 8 Ali S, Mumar S, Kalam K, Raja K, Baqi S (2014). "Prevalence, clinical presentation and treatment outcome of cryptosporidiosis in immunocompetent adult patients presenting with acute diarrhoea". J Pak Med Assoc. 64 (6): 613–8. PMID 25252476.
All 58 patients reported resolution of diarrhoea after 7 days of treatment with nitazoxanide. However, 40 (70.1%) patients reported recurrence of diarrhoea within 6 weeks of treatment. ... Our study demonstrates a high prevalence of cryptosporidiosis in immunocompetent adult patients. Nitazoxanide is the recommended antimicrobial drug for cryptosporidiosis. ... The frequency of cryptosporidiosis has not been well-defined. About 30% of the adult population of the United States are seropositive with over 10,500 cases reported in 2008. ... Although we gave 7 days of therapy and a satisfactory treatment response was obtained in the short term, there was a high recurrence rate.21 Paromomycin and/or azithromycin in combination with nitazoxanide have been tested in double blind randomized trials for the treatment of cryptosporidiosis in immunocomprised patients such as those with HIV/AIDS, and the results have been encouraging.18,22,23
- ↑ Hawkins S, Thomas R, Teasdale C (1987). "Acute pancreatitis: a new finding in cryptosporidium enteritis". Br Med J (Clin Res Ed). 294 (6570): 483–4. PMC 1245527
 . PMID 3103738. doi:10.1136/bmj.294.6570.483-a.
. PMID 3103738. doi:10.1136/bmj.294.6570.483-a. - ↑ Graczyk TK, Fayer R, Knight R, et al. (2000). "Mechanical transport and transmission of Cryptosporidium parvum oocysts by wild filth flies". Am. J. Trop. Med. Hyg. 63 (3–4): 178–83. PMID 11388511.
- ↑ Carreno RA, Martin DS, Barta JR (November 1999). "Cryptosporidium is more closely related to the gregarines than to coccidia as shown by phylogenetic analysis of apicomplexan parasites inferred using small-subunit ribosomal RNA gene sequences". Parasitol. Res. 85 (11): 899–904. PMID 10540950. doi:10.1007/s004360050655. Archived from the original on 20 March 2001.
- ↑ Abrahamsen, M. S.; Templeton, TJ; Enomoto, S; Abrahante, JE; Zhu, G; Lancto, CA; Deng, M; Liu, C; et al. (2004). "Complete Genome Sequence of the Apicomplexan, Cryptosporidium parvum". Science. 304 (5669): 441–5. PMID 15044751. doi:10.1126/science.1094786.
- ↑ Xu, P.; Widmer, G; Wang, Y; Ozaki, LS; Alves, JM; Serrano, MG; Puiu, D; Manque, P; et al. (2004). "The genome of Cryptosporidium hominis". Nature. 431 (7012): 1107–12. PMID 15510150. doi:10.1038/nature02977.
- ↑ Carpenter, Colleen; Fayer, Ronald; Trout, James; Beach, Michael J. (1999). "Chlorine Disinfection of Recreational Water for Cryptosporidium parvum". Emerging Infectious Diseases. 5 (4): 579–84. PMC 2627758
 . PMID 10458969. doi:10.3201/eid0504.990425.
. PMID 10458969. doi:10.3201/eid0504.990425. - ↑ Teresa Ortega, María; Vergara, Alberto; Guimbao, Joaquín; Clavel, Antonio; Gavín, Patricia; Ruiz, Andrés (2006). "Brote de diarrea y transmisión de Cryptosporidium hominis asociados al uso de pañal en niños" [Cryptosporidium hominis diarrhea outbreak and transmission linked to diaper infant use]. Medicina Clínica (in Spanish). 127 (17): 653–6. PMID 17169283. doi:10.1016/S0025-7753(06)72352-1.
- ↑ Kelley, Amy S. (2014). "Defining "Serious Illness"". Journal of Palliative Medicine. 17 (9): 985. doi:10.1089/jpm.2014.0164.
- ↑ "Chlorine Disinfection of Recreational Water for Cryptosporidium parvum". CDC. Retrieved 6 May 2007.
- 1 2 3 "Cryptosporidiosis". Gideon. 23 February 2009.
Trial subscription required to access
- 1 2 3 4 5 6 7 8 9 Ryan, Kenneth J.; Ray, C. George (2004). Sherris Medical Microbiology: An Introduction to Infectious Disease (4th ed.). New York: McGraw-Hill. pp. 727–730.
- 1 2 3 4 Chen XM, Keithly JS, Paya CV, LaRusso NF (May 2002). "Cryptosporidiosis". N. Engl. J. Med. 346 (22): 1723–31. PMID 12037153. doi:10.1056/NEJMra013170.
- 1 2 3 4 5 6 Murray, Patrick R., Ken S. Rosenthal, and Michael A. Pfaller. Medical Microbiology. 5th ed. Philadelphia: Elsevier Inc., 2005: 855–856.
- 1 2 3 4 5 Winn Jr., Washington; Allen, Stephen; Janda, William; Koneman, Elmer; Procop, Gary; Schreckenberger, Paul; Woods, Gail (2006). Koneman’s Color Atlas and Textbook of Diagnostic Microbiology (6th ed.). Philadelphia: Lippincott Williams & Wilkins. pp. 1267–70.
- 1 2 3 4 5 6 Chen W, Harp JA, Harmsen AG (April 2003). "Cryptosporidium parvum infection in gene-targeted B cell-deficient mice". J. Parasitol. 89 (2): 391–3. PMID 12760662. doi:10.1645/0022-3395(2003)089[0391:CPIIGB]2.0.CO;2.
- 1 2 3 4 5 Brooks, Geo. F.; Butel, Janet S.; Morse, Stephen A. (2004). Jawetz, Melnick, & Adelberg’s Medical Microbiology (23rd ed.). New York: Lange Medical Books/McGraw Hill. pp. 684–5.
- ↑ Chappell CL, Okhuysen PC, Langer-Curry RC, Lupo PJ, Widmer G, Tzipori S (2015). "Cryptosporidium muris: infectivity and illness in healthy adult volunteers". Am. J. Trop. Med. Hyg. 92 (1): 50–5. PMC 4347390
 . PMID 25311695. doi:10.4269/ajtmh.14-0525.
. PMID 25311695. doi:10.4269/ajtmh.14-0525. C. muris-infected subjects shed oocysts longer than occurred with other species studied in healthy volunteers. Three volunteers shed oocysts for 7 months. ... Thus, healthy adults are susceptible to C. muris, which can cause mild diarrhea and result in persistent, asymptomatic infection.
- ↑ Clark, Douglas P. (1 October 1999). "New Insights into Human Cryptosporidiosis". Clinical Microbiology Reviews. 12 (4): 554–563. ISSN 0893-8512.
- ↑ Okhuysen, Pablo C.; Chappell, Cynthia L.; Sterling, Charles R.; Jakubowski, Walter; DuPont, Herbert L. (1 February 1998). "Susceptibility and Serologic Response of Healthy Adults to Reinfection with Cryptosporidium parvum". Infection and Immunity. 66 (2): 441–443. ISSN 0019-9567.
- ↑ "The Interim Enhanced Surface Water Treatment Rule – What Does it Mean to You?" (PDF). USEPA. Archived from the original (pdf) on 28 September 2007. Retrieved 6 May 2007.
- ↑ Korich DG, Mead JR, Madore MS, Sinclair NA, Sterling CR (May 1990). "Effects of ozone, chlorine dioxide, chlorine, and monochloramine on Cryptosporidium parvum oocyst viability". Appl. Environ. Microbiol. 56 (5): 1423–8. PMC 184422
 . PMID 2339894.
. PMID 2339894. - ↑ Rochelle, PAUL A.; Fallar, D; Marshall, MM; Montelone, BA; Upton, SJ; Woods, K (September–October 2004). "Irreversible UV inactivation of Cryptosporidium spp. despite the presence of UV repair genes". J Eukaryot Microbiol. 51 (5): 553–62. PMID 15537090. doi:10.1111/j.1550-7408.2004.tb00291.x.
- ↑ "Ultraviolet Disinfection and Treatment". WaterResearchFoundation (formerly AwwaRF). Archived from the original on 24 January 2009. Retrieved 6 May 2007.
- ↑ "Boil water warning 'precaution'". BBC. 2 September 2008. Retrieved 7 September 2009.
- ↑ "Boil water 'into January' warning". BBC. 30 November 2005. Retrieved 7 September 2009.
- 1 2 John, David T. and William A. Petri, Jr. Markell and Voge’s Medical Parasitology. 9th ed. Philadelphia: Elsevier Inc., 2006: 68–71.
- ↑ "Control measures for Outbreaks — Intensified Cryptosporidiosis (Crypto) Control Measures for the Child Care Setting". US Centers for Disease Control and Prevention.
- 1 2 3 4 5 6 7 8 Cabada MM, White AC, Venugopalan P, Sureshbabu J (18 August 2015). Bronze MS, ed. "Cryptosporidiosis Treatment & Management". Medscape. WebMD. Retrieved 8 January 2016.
Infection may improve with nutritional supplementation, particularly with regimens including zinc or glutamine. ... Nitazoxanide significantly shortens the duration of diarrhea and can decrease the risk of mortality in malnourished children.[22] Trials have also demonstrated efficacy in adults.[26, 27] ... Use of partially active antiparasitic drugs (eg, nitazoxanide or paromomycin combined with azithromycin) should be considered along with initiating antiretroviral therapy. ... Symptomatic therapy includes replacement of fluids, provision of appropriate nutrition, and treatment with antimotility agents. ... Replacement of fluids and electrolytes is the critically important first step in the management of cryptosporidiosis, particularly in patients with large diarrheal losses. Fluids should include sodium, potassium, bicarbonate, and glucose.
- 1 2 3 4 5 Abubakar I, Aliyu SH, Arumugam C, Hunter PR, Usman NK (January 2007). "Prevention and treatment of cryptosporidiosis in immunocompromised patients". Cochrane Database Syst Rev (1): CD004932. PMID 17253532. doi:10.1002/14651858.CD004932.pub2.
The results indicate that nitaxozanide reduces the load of parasites and may be useful in immunocompetent individuals. Due to the seriousness of the potential outcomes of cryptosporidiosis, the use of nitaxozanide should be considered in immunocompromised patients. The absence of effective therapy highlights the need to ensure that infection is avoided. ... For HIV-infected persons, highly active antiretroviral therapy (HAART) is the mainstay of preventing and managing cryptosporidiosis. HAART can lead to complete resolution of clinical symptoms and oocysts (Grube 1997; Maggi 2000; Miao 2000). This intervention is not available for HIV patients who are failing HAART or those unable to access HAART in developing countries. Among these immunocompromised persons without the option of an effective treatment for the underlying disease, supportive management, including rehydration therapy, electrolyte replacement, and anti-motility agents will remain the only alternatives for care until better drugs emerge.
- 1 2 Sparks H, Nair G, Castellanos-Gonzalez A, White AC (2015). "Treatment of Cryptosporidium: What We Know, Gaps, and the Way Forward". Curr Trop Med Rep. 2 (3): 181–187. PMID 26568906. doi:10.1007/s40475-015-0056-9.
- ↑ Gilson M.D., Ian; Buggy, Brian P. M.D. (October 1996). "Cryptosporidiosis in Patients with HIV Disease: Is It Safe to Drink the Water?". HIV Newsline. San Francisco General Hospital.
- ↑ Gargala G (September 2008). "Drug treatment and novel drug target against Cryptosporidium". Parasite. 15 (3): 275–81. PMID 18814694. doi:10.1051/parasite/2008153275.
- ↑ Gatei W, Wamae CN, Mbae C, et al. (July 2006). "Cryptosporidiosis: prevalence, genotype analysis, and symptoms associated with infections in children in Kenya". Am. J. Trop. Med. Hyg. 75 (1): 78–82. PMID 16837712.
- ↑ Lozano, R (15 December 2012). "Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010.". Lancet. 380 (9859): 2095–128. PMID 23245604. doi:10.1016/S0140-6736(12)61728-0.
- ↑ Xiao, L.; Fayer, R.; Ryan, U.; Upton, S. J. (2004). "Cryptosporidium Taxonomy: Recent Advances and Implications for Public Health". Clinical Microbiology Reviews. 17 (1): 72–97. ISSN 0893-8512. PMC 321466
 . PMID 14726456. doi:10.1128/CMR.17.1.72-97.2004.
. PMID 14726456. doi:10.1128/CMR.17.1.72-97.2004. - ↑ Lassen B, Ståhl M, Enemark HL (2014). "Cryptosporidiosis - an occupational risk and a disregarded disease in Estonia." (PDF). Acta Vet. Scand. 56: 36. PMC 4089559
 . PMID 24902957. doi:10.1186/1751-0147-56-36.
. PMID 24902957. doi:10.1186/1751-0147-56-36. - ↑ Corso P, Kramer M, Blair K, Addiss D, Davis J, Haddix A (2003). "Costs of Illness in the 1993 Waterborne Cryptosporidium Outbreak, Milwaukee, Wisconsin". Emerg Infect Dis. 9 (4): 426–31. PMC 2957981
 . PMID 12702221. doi:10.3201/eid0904.020417.
. PMID 12702221. doi:10.3201/eid0904.020417. - ↑ "Cryptosporidium". CBC News. 23 June 2004. Archived from the original on 1 March 2011. Retrieved 19 April 2011.
- ↑ "Waterborne Cryptosporidiosis Outbreak, North Battleford, Ssaskatchewan, Spring 2001". Public Health Agency of Canada. 15 November 2001. Retrieved 25 January 2008.
- ↑ "State Health Department Issues Update on Seneca Lake State Park Gastrointestinal Outbreak". New York State Health Dept. Retrieved 29 September 2006.
- ↑ RTÉ News — Galway water now safer than ever — HSE
- ↑ Yahoo.com, Cryptosporidium outbreak hits the West
- ↑ Northampton Chronicle and Echo
- ↑ Anglian Water-lifting of boil notice
- ↑ Crypto spreads to private pools — WFAA-TV. Retrieved 13 August 2008.
- ↑ Gym closes pool in danger bug alert
- ↑ Mawer, Jessica (20 May 2010). "Woree, Gordonvale residents advised to boil drinking water". ABC Online. Retrieved 19 April 2011.
- ↑ "Smittskyddsinstitutets arbete med det vattenburna utbrottet av Cryptosporidium i Östersund" (in Swedish). Smittskyddsinstitutet. Retrieved 19 April 2011.
- ↑ Sjöö, Patrick (13 December 2010). "Kommunens parasitenkät avslutas". Östersunds-Posten (in Swedish). Retrieved 19 April 2011.
- ↑ "Boil water notice after Cryptosporidiosis outbreak in Co Roscommon". RTÉ News. 15 May 2013.
- ↑ "13 people treated following Roscommon water pollution". RTÉ News. 17 May 2013.
- ↑ Thirst (1998) IMDB
- White, A. Clinton, Jr. (2005). "Cryptosporidiosis". In Mandell, G; et al. Principles and Practice of Infectious Diseases (6th ed.). Elsevier. pp. 3215–28.
- Upton, Steve J. (12 September 2003). "Basic Biology of Cryptosporidium" (Website). Kansas State University: Parasitology Laboratory.
- S.J. Brands (Compiler) (2000). "The Taxonomicon & Systema Naturae" (Website database). Taxon: Genus Cryptosporidium. Universal Taxonomic Services, Amsterdam, The Netherlands.
- Heymann, David (2015). Control of communicable diseases manual : an official report of the American Public Health Association. APHA Press, the American Public Health Association. ISBN 9780875530185.
External links
- Cryptosporidiosis — Centers for Disease Control and Prevention
- Aquatics International Article Regarding Infection via Spray Parks
- CryptoDB: The Cryptosporidium Genome Resource