Crotamine
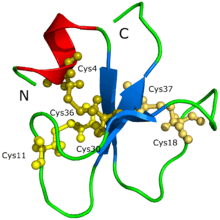
Crotamine is a toxin present in the venom of the South American rattlesnake (Crotalus durissus terrificus). It is a 42-residue long protein containing 11 basic residues (9 lysines, 2 arginines) and 6 cysteines. It has also been isolated from the venom of North American prairie rattlesnake, Crotalus viridis viridis. It was first isolated and purified by Brazilian scientist José Moura Gonçalves, and later intensively studied by his group of collaborators at the Medical School of Ribeirão Preto of the University of São Paulo.
Biological function
Crotamine has a number of biological actions: it acts on cell membrane's sodium channels, is slightly analgesic and is myotoxic, i.e., it penetrates the cells of muscles and promotes necrosis. Crotamine is homologous with other venom myotoxins and is similar to α-,β-defensins.
Biochemistry and mechanism
The amino acid sequence, YKQCHKKGGHCFPKEKICLPPSSDFGKMDCRWRWKCCKKGS—G, and the 3D molecular structure of crotamine have already been determined.
The protein structure of crotamine could not be initially determined through protein crystallization nor X-ray diffraction.[1] It was speculated that the difficulty was because crotamine has so many isoforms, leading to the formation of aggregates and different possible conformations of the protein. The structure and the shape of the protein was proposed through a 3D model generated by Siqueira et al. (2002) based on computational calculations that were supported with intensive molecular dynamics simulations and homology modeling procedures. Afterwards, Nicastro et al. (2003) discovered the structure of crotamine through nuclear magnetic resonance spectroscopy. Crotamine has a topology that was never before seen in active toxins that target ion channels; the protein is composed of a short N-terminal alpha helix, a type of protein formation, and a small antiparallel triple-stranded beta-sheet, another type of protein formation, arranged in an ab1b2b3 topology. Crotamine has similar structural fold conformations to the human b-defensin family as well as identical disulfide bridges arrangement.[2]
[Figure needed]
The gene and chromosome location responsible for its synthesis have been identified by the group led by Gandhi Rádis-Baptista, working at the Instituto Butantan, in São Paulo, Brazil. The mRNA has about 340 nucleotides and codifies a pre-crotamine, including the signal peptide, the mature crotamine, and a final lysine.
The Crotamine gene was the first gene to be mapped on a snake chromosome.[3] The gene responsible for coding the crotamine protein is labeled as Crt-p1 and its base pair sequence length is about 1.1kbp or 1100 bp. It was reported that the crotamine gene was isolated twice from two different specimens, one in a method that resulted in a gene size of 1.8 kbp and in the other specimen a gene size of 1.1 kbp.[4] The gene has been previously isolated in the C. durissus terrificus genome and the protein itself belongs to a group of small basic polypeptide myotoxins (SBPM). The contents of Crotalus venoms can vary according to subspecies and geographical location.[5] The Crt-p1 gene, as described by Radis-Bastista et al. 2003, consists of about three exons separated by one short phase-2 (140 bp) and one long phase-1 (900 bp) intron. Exon 1 codes for the first 19 amino acids of the signal peptide and includes the 5’-untranslated region. Exon 2 codes 39 amino acids to the mature crotamine and three signal peptide amino acids. Exon 3 codes for the terminal lysine and the last three amino acids of the mature toxin. Research on SBPM amino acid sequences among different Crotalus species has revealed a high degree of likeness ranging from 83% - 98%.[6][7]
The amino acid code of proteins in the small basic polypeptide myotoxin family, which includes crotamine, have been sequenced. They were found to be similar with an average of 83% divergence. A crotamine amino acid sequence was compared to that of cloned DNA of myotoxin a, (the myotoxin used to model how SBPMs work). In the comparison, exon coding regions including the mature myotoxin and the signal peptide were 98% and 100% similar, respectively. The untranslated regions for 5’ and 3’ between the sample and the myotoxin a cDNA was 60% and 80%, respectively. When comparing the amino acid sequences of other proteins not in the SBPM family found in snake venoms, there is usually large divergence. When looking at the SBPM proteins, they have high similarity between different subspecies of the Crotalus genus and between different individuals of the same subspecies. This indicates, according to the Radis-Batista et al. 2003 study, that the crotamine gene and other SBPM genes have evolved recently.
References
- ↑ Boni-Mitake, M., Ra´dis-Baptistam , G., Oguiura, N., 2005. New view on crotamine, a small basic polypeptide myotoxin from South American rattlesnake venom. Toxicon 46, 363-370. < http://www.ipen.br/biblioteca/2005/11186.pdf>
- ↑ Boni-Mitake, M., Ra´dis-Baptistam , G., Oguiura, N., 2005. New view on crotamine, a small basic polypeptide myotoxin from South American rattlesnake venom. Toxicon 46, 363-370. < http://www.ipen.br/biblioteca/2005/11186.pdf>
- ↑ Boni-Mitake, M., Ra´dis-Baptistam , G., Oguiura, N., 2005. New view on crotamine, a small basic polypeptide myotoxin from South American rattlesnake venom. Toxicon 46, 363-370. < http://www.ipen.br/biblioteca/2005/11186.pdf>.
- ↑ Samejima, Y., Aoki, Y., Mebs, D., 1991. Amino acid sequence of a myotoxin from venom of the eastern diamondback rattlesnake(Crotalus adamanteus). Toxicon 29, 461–468.
- ↑ Schenberg, S., 1959. Geographical pattern of crotamine distribution in the same rattlesnake subspecies. Science 129, 1361–1363.
- ↑ Samejima, Y., Aoki, Y., Mebs, D., 1991. Amino acid sequence of a myotoxin from venom of the eastern diamondback rattlesnake(Crotalus adamanteus). Toxicon 29, 461–468.
- ↑ Ownby, C., 1998. Structure, function and biophysical aspects of the myotoxins from snake venoms. J. Toxicol. Toxin. Rev. 17, 213–238.
Further reading
- Siqueira, A.M., Martins, N.F., Lima, M.E. de, Diniz, C.R., Cartier, A., Brown, D., Maigret, B., 2002. A proposed 3D structure for crotamine based on homology building, molecular simulations and circular ichroism. J. Mol. Graph. Model. 20, 389–398.
- Gonçalves JM, Deutsch HF. Ultracentrifugal and zone electrophoresis studies of some crotalidae venoms. Arch Biochem Biophys. 1956 Feb;60(2):402-11. doi:10.1016/0003-9861(56)90444-1 PMID 13292919
- Giglio JR. Analytical studies on crotamine hydrochloride. Anal Biochem. 1975 Nov;69(1):207-21. PMID 2030
- Laure CJ. The primary structure of crotamine. Hoppe Seylers Z Physiol Chem. 1975 Feb;356(2):213-5. German. PMID 1176086
- De Lucca FL, Imaizumi MT, Haddad A. Characterization of ribonucleic acids from the venom glands of Crotalus durissus terrifucus (Ophidia, Reptilia) after manual extraction of the venom. Studies on template activity and base composition. Biochem J. 1974 Apr;139(1):151-6. PMID 4463939
- Ownby, C. L., Cameron, M. S., and Tu, A. T. (1976) Isolation of myotoxin component from rattlesnake (Crotalus viridis viridis) venom. Am. J. Pathol. 85, 149–166
- Rádis-Baptista, G., Oguiura, N., Hayashi, M. A. F., Camargo, M. E., Grego, K. F., Oliveira, E. B., and Yamane, T. (1999) Nucleotide sequence of crotamine isoform precursors from a single South American rattlesnake (Crotalus durissus terrificus). Toxicon 37, 973–984
- Alexandre Kerkis, Irina Kerkis, Gandhi Rádis-Baptista, Eduardo B. Oliveira, Angela M. Vianna-Morgante, Lygia V. Pereira, and Tetsuo Yamane. Crotamine is a novel cell-penetrating protein from the venom of rattlesnake Crotalus durissus terrificus. The FASEB Journal Express Article doi:10.1096/fj.03-1459fje. Published online July 1, 2004
- Radis-Baptista G, Kubo T, Oguiura N, Prieto da Silva AR, Hayashi MA, Oliveira EB, Yamane T. Identification of crotasin, a crotamine-related gene of Crotalus durissus terrificus. Toxicon. 2004 Jun 1;43(7):751-9. PMID 15284009
- Radis-Baptista G, Kubo T, Oguiura N, Svartman M, Almeida TM, Batistic RF, Oliveira EB, Vianna-Morgante AM, Yamane T. Structure and chromosomal localization of the gene for crotamine, a toxin from the South American rattlesnake, Crotalus durissus terrificus. Toxicon. 2003 Dec;42(7):747-52. PMID 14757205
- Nicastro G, Franzoni L, de Chiara C, Mancin AC, Giglio JR, Spisni A. Solution structure of crotamine, a Na+ channel affecting toxin from Crotalus durissus terrificus venom. Eur J Biochem. 2003 May;270(9):1969-79. PMID 12709056
- Mouhat S, Jouirou B, Mosbah A, De Waard M, Sabatier JM. Diversity of folds in animal toxins acting on ion channels. Biochem J. 2004 Mar 15;378(Pt 3):717-26. PMID 14674883
External links
- Nucleotide sequence and translation for crotasin. Entrez Database. National Center for Biotechnology Information.