Coxsackie virus and adenovirus receptor
Coxsackievirus and adenovirus receptor (CAR) is a protein that in humans is encoded by the CXADR gene.[3][4][5] The protein encoded by this gene is a type I membrane receptor for group B coxsackie viruses and subgroup C adenoviruses. CAR protein is expressed in several tissues, including heart, brain, and, more generally, epithelial and endothelial cells. In cardiac muscle, CAR is localized to intercalated disc structures, which electrically and mechanically couple adjacent cardiomyocytes. CAR plays an important role in the pathogenesis of myocarditis, dilated cardiomyopathy, and in arrhythmia susceptibility following myocardial infarction or myocardial ischemia.
Structure
Human CAR protein has a theoretical molecular weight of 40.0 kDa and is composed of 365 amino acids.[6] The human CAR gene (CXADR) is found on chromosome 21. Alternative splicing is known to produce at least 2 splice variants known as hCAR1 and hCAR2 and are each composed of at least 7 exons. Pseudogenes of this gene are found on chromosomes 15, 18, and 21.[5]
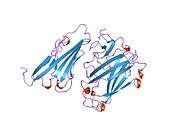
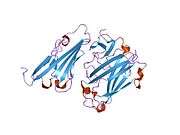
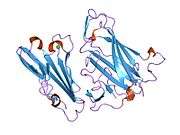
CAR is a transmembrane bound protein with two Ig-like extracellular domains, a transmembrane domain, a cytoplasmic domain, and two N-linked glycosylation sites. CAR contains two disulfide bonded loops (residues 35-130 and 155-220).[7] The N-terminal segment comprises the two extracellular domains (D1 and D2). D1 is most distal from the membrane and contains a V/Ig-like fold whereas D2 is more proximal. The cytoplasmic tail of CAR contains the amino acids GSIV, which is characterized as a class 1 PDZ binding motif for interacting with proteins containing PDZ domains.[8]
The protein is found to be expressed in various regions of the body including the heart, brain, and, more generally, epithelial and endothelial cells. Moreover, CAR expression is not found in normal or tumor cell lines. Expression of CAR in endothelial cells can be regulated by treatment with drugs.[9][10]
Function
It functions as a homophilic and heterophilic cell adhesion molecule through its interactions with extracellular matrix glycoproteins such as: fibronectin, agrin, laminin-1 and tenascin-R.[11] In addition, it is thought to regulate the cytoskeleton through interactions with actin and microtubules. Moreover, its cytoplasmic domain contains putative phosphorylation sites and a PDZ-interaction motif which suggests a scaffolding role.
Cardiac
CAR is essential for normal development of cardiomyocytes. The expression of CAR is high in developing tissues, including the heart and brain; postnatally it is expressed in epithelial cells and in adult cardiac muscle, it is localized at intercalated discs.[12] Knocking out CAR is embryonic lethal by day 11.5, coordinate with severe cardiac muscle abnormalities including left ventricular hyperplasia, sinuatrial valve abnormalities, pericardial edema, thoracic hemorrhaging, myocardial wall degeneration, regional apoptosis, reduced density and disorganization of myofibrils, and enlarged mitochondria.[13][14][15] Cardiomyocyte-specific deletion of CAR after embryonic day 11 had no noticeable effect on development and postnatal life, suggesting that CAR is critical during a temporal window of cardiac development.[15]
It is clear from studies employing transgenesis that CAR function at intercalated discs in cardiac muscle is critical for normal heart function. Cardiac-specific knockout of CAR causes first degree block or complete block in the propagation of electrical conduction in the AV node. This was coordinate with the loss of connexin-45 at cell-cell junctions on the sarcolemmal membranes of AV node cells. Mice eventually developed cardiomyopathy associated with intercalated disc disorganization and loss of cardiomyocyte beta-catenin and ZO-1 expression; studies also showed that CAR, and connexin-45 form a protein complex that requires the PDZ-binding motif on CAR for proper formation. Moreover, CAR is required for normal localization of connexin-45, beta-catenin and ZO-1 at intercalated discs.[16]
Studies from human hearts have shown that lower expression of CXADR mRNA is associated with a risk allele at chromosome 21q21, which may in fact predispose hearts to arrhythmias. To discern the mechanistic underpinnings, hearts from heterozygous CAR knockout mice subjected to acute myocardial ischemia were evaluated and showed slowed ventricular conduction, earlier onset of ventricular arrhythmias, and increased susceptibility to arrhythmias. These findings were coordinate with a reduction in magnitude of the sodium current at intercalated discs; interestingly, CAR coprecipitated with NaV1.5, which may provide a mechanistic link to this finding.[17]
Neural and lymphatic
CAR is strongly expressed in the developing central nervous system where it is thought to mediate neurite outgrowth. In contrast,expression of CAR is undetectable in the adult nervous system.[11]
It has also been shown that CAR is critical for the development of lymphatic vasculature and in forming lymphatic endothelial cell-cell junctions.[18]
Clinical Significance
CAR is a receptor for both Coxsackie B virus and adenovirus 2 and 5, which are structurally distinct.[19]
In patients with myocarditis or dilated cardiomyopathy, elevated Coxsackie B2 viral nucleic acids have been detected in myocardial biopsy samples.[20] Adenoviral genomic DNA has also been detected in myocardial biopsies of patients with idiopathic cardiomyopathy, or impaired left ventricular function of unknown origin.[21] Patients exhibiting sudden death from acute myocardial infarction had a higher proportion of active coxsackie B virus infection relative to matched controls, which was coordinate with disrupted sarcolemmal localization of dystrophin, suggesting that enteroviral infection may worsen the outcome of patients with acute myocardial infarction.[22]
A role for CAR in arrhythmia susceptibility and ventricular fibrillation after myocardial infarction was shown in that CXADR lies near the 21q21 locus, which is strongly associated with these insults.[17][23][24][25]
Interactions
CAR has been shown to interact with: MAGI-1b,[8] PICK1,[8] PSD-95,[8] ZO-1,[26] NaV1.5[17]
References
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW (Feb 1997). "Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5". Science. 275 (5304): 1320–3. PMID 9036860. doi:10.1126/science.275.5304.1320.
- ↑ Tomko RP, Xu R, Philipson L (Apr 1997). "HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses". Proceedings of the National Academy of Sciences of the United States of America. 94 (7): 3352–6. PMC 20373
 . PMID 9096397. doi:10.1073/pnas.94.7.3352.
. PMID 9096397. doi:10.1073/pnas.94.7.3352. - 1 2 "Entrez Gene: CXADR coxsackie virus and adenovirus receptor".
- ↑ "Protein sequence of human CXADR (Uniprot ID: P78310)". Cardiac Organellar Protein Atlas Knowledgebase (COPaKB). Retrieved 14 July 2015.
- ↑ Tomko RP, Xu R, Philipson L (Apr 1997). "HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses". Proceedings of the National Academy of Sciences of the United States of America. 94 (7): 3352–6. PMC 20373
 . PMID 9096397. doi:10.1073/pnas.94.7.3352.
. PMID 9096397. doi:10.1073/pnas.94.7.3352. - 1 2 3 4 Excoffon KJ, Hruska-Hageman A, Klotz M, Traver GL, Zabner J (Sep 2004). "A role for the PDZ-binding domain of the coxsackie B virus and adenovirus receptor (CAR) in cell adhesion and growth". Journal of Cell Science. 117 (Pt 19): 4401–9. PMID 15304526. doi:10.1242/jcs.01300.
- ↑ Funke C, Farr M, Werner B, Dittmann S, Uberla K, Piper C, Niehaus K, Horstkotte D (Aug 2010). "Antiviral effect of Bosentan and Valsartan during coxsackievirus B3 infection of human endothelial cells". The Journal of General Virology. 91 (Pt 8): 1959–70. PMID 20392896. doi:10.1099/vir.0.020065-0.
- ↑ Werner B, Dittmann S, Funke C, Überla K, Piper C, Niehaus K, Horstkotte D, Farr M (Apr 2014). "Effect of lovastatin on coxsackievirus B3 infection in human endothelial cells". Inflammation Research. 63 (4): 267–76. PMID 24316867. doi:10.1007/s00011-013-0695-z.
- 1 2 Patzke C, Max KE, Behlke J, Schreiber J, Schmidt H, Dorner AA, Kröger S, Henning M, Otto A, Heinemann U, Rathjen FG (Feb 2010). "The coxsackievirus-adenovirus receptor reveals complex homophilic and heterophilic interactions on neural cells". The Journal of Neuroscience. 30 (8): 2897–910. PMID 20181587. doi:10.1523/JNEUROSCI.5725-09.2010.
- ↑ Kashimura T, Kodama M, Hotta Y, Hosoya J, Yoshida K, Ozawa T, Watanabe R, Okura Y, Kato K, Hanawa H, Kuwano R, Aizawa Y (Mar 2004). "Spatiotemporal changes of coxsackievirus and adenovirus receptor in rat hearts during postnatal development and in cultured cardiomyocytes of neonatal rat". Virchows Archiv. 444 (3): 283–92. PMID 14624362. doi:10.1007/s00428-003-0925-9.
- ↑ Asher DR, Cerny AM, Weiler SR, Horner JW, Keeler ML, Neptune MA, Jones SN, Bronson RT, Depinho RA, Finberg RW (Jun 2005). "Coxsackievirus and adenovirus receptor is essential for cardiomyocyte development". Genesis. 42 (2): 77–85. PMID 15864812. doi:10.1002/gene.20127.
- ↑ Dorner AA, Wegmann F, Butz S, Wolburg-Buchholz K, Wolburg H, Mack A, Nasdala I, August B, Westermann J, Rathjen FG, Vestweber D (Aug 2005). "Coxsackievirus-adenovirus receptor (CAR) is essential for early embryonic cardiac development". Journal of Cell Science. 118 (Pt 15): 3509–21. PMID 16079292. doi:10.1242/jcs.02476.
- 1 2 Chen JW, Zhou B, Yu QC, Shin SJ, Jiao K, Schneider MD, Baldwin HS, Bergelson JM (Apr 2006). "Cardiomyocyte-specific deletion of the coxsackievirus and adenovirus receptor results in hyperplasia of the embryonic left ventricle and abnormalities of sinuatrial valves". Circulation Research. 98 (7): 923–30. PMID 16543498. doi:10.1161/01.RES.0000218041.41932.e3.
- ↑ Lim BK, Xiong D, Dorner A, Youn TJ, Yung A, Liu TI, Gu Y, Dalton ND, Wright AT, Evans SM, Chen J, Peterson KL, McCulloch AD, Yajima T, Knowlton KU (Aug 2008). "Coxsackievirus and adenovirus receptor (CAR) mediates atrioventricular-node function and connexin 45 localization in the murine heart". The Journal of Clinical Investigation. 118 (8): 2758–70. PMC 2467382
 . PMID 18636119. doi:10.1172/JCI34777.
. PMID 18636119. doi:10.1172/JCI34777. - 1 2 3 Marsman RF, Bezzina CR, Freiberg F, Verkerk AO, Adriaens ME, Podliesna S, Chen C, Purfürst B, Spallek B, Koopmann TT, Baczko I, Dos Remedios CG, George AL, Bishopric NH, Lodder EM, de Bakker JM, Fischer R, Coronel R, Wilde AA, Gotthardt M, Remme CA (Feb 2014). "Coxsackie and adenovirus receptor is a modifier of cardiac conduction and arrhythmia vulnerability in the setting of myocardial ischemia". Journal of the American College of Cardiology. 63 (6): 549–59. PMC 3926969
 . PMID 24291282. doi:10.1016/j.jacc.2013.10.062.
. PMID 24291282. doi:10.1016/j.jacc.2013.10.062. - ↑ Mirza M, Pang MF, Zaini MA, Haiko P, Tammela T, Alitalo K, Philipson L, Fuxe J, Sollerbrant K (2012). "Essential role of the coxsackie- and adenovirus receptor (CAR) in development of the lymphatic system in mice". PLOS ONE. 7 (5): e37523. PMC 3356332
 . PMID 22624044. doi:10.1371/journal.pone.0037523.
. PMID 22624044. doi:10.1371/journal.pone.0037523. - ↑ Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW (Feb 1997). "Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5". Science. 275 (5304): 1320–3. PMID 9036860. doi:10.1126/science.275.5304.1320.
- ↑ Bowles NE, Richardson PJ, Olsen EG, Archard LC (May 1986). "Detection of Coxsackie-B-virus-specific RNA sequences in myocardial biopsy samples from patients with myocarditis and dilated cardiomyopathy". Lancet. 1 (8490): 1120–3. PMID 2871380. doi:10.1016/s0140-6736(86)91837-4.
- ↑ Pauschinger M, Bowles NE, Fuentes-Garcia FJ, Pham V, Kühl U, Schwimmbeck PL, Schultheiss HP, Towbin JA (Mar 1999). "Detection of adenoviral genome in the myocardium of adult patients with idiopathic left ventricular dysfunction". Circulation. 99 (10): 1348–54. PMID 10077520. doi:10.1161/01.cir.99.10.1348.
- ↑ Andréoletti L, Ventéo L, Douche-Aourik F, Canas F, Lorin de la Grandmaison G, Jacques J, Moret H, Jovenin N, Mosnier JF, Matta M, Duband S, Pluot M, Pozzetto B, Bourlet T (Dec 2007). "Active Coxsackieviral B infection is associated with disruption of dystrophin in endomyocardial tissue of patients who died suddenly of acute myocardial infarction". Journal of the American College of Cardiology. 50 (23): 2207–14. PMID 18061067. doi:10.1016/j.jacc.2007.07.080.
- ↑ Marsman RF, Wilde AA, Bezzina CR (Feb 2011). "Genetic predisposition for sudden cardiac death in myocardial ischaemia: the Arrhythmia Genetics in the NEtherlandS study". Netherlands Heart Journal. 19 (2): 96–100. PMC 3040308
 . PMID 21461030. doi:10.1007/s12471-010-0070-4.
. PMID 21461030. doi:10.1007/s12471-010-0070-4. - ↑ Bezzina CR, Pazoki R, Bardai A, Marsman RF, de Jong JS, Blom MT, Scicluna BP, Jukema JW, Bindraban NR, Lichtner P, Pfeufer A, Bishopric NH, Roden DM, Meitinger T, Chugh SS, Myerburg RJ, Jouven X, Kääb S, Dekker LR, Tan HL, Tanck MW, Wilde AA (Aug 2010). "Genome-wide association study identifies a susceptibility locus at 21q21 for ventricular fibrillation in acute myocardial infarction". Nature Genetics. 42 (8): 688–91. PMC 3966292
 . PMID 20622880. doi:10.1038/ng.623.
. PMID 20622880. doi:10.1038/ng.623. - ↑ Chopra N, Knollmann BC (May 2011). "Genetics of sudden cardiac death syndromes". Current Opinion in Cardiology. 26 (3): 196–203. PMC 3145336
 . PMID 21430528. doi:10.1097/HCO.0b013e3283459893.
. PMID 21430528. doi:10.1097/HCO.0b013e3283459893. - ↑ Cohen CJ, Shieh JT, Pickles RJ, Okegawa T, Hsieh JT, Bergelson JM (Dec 2001). "The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction". Proceedings of the National Academy of Sciences of the United States of America. 98 (26): 15191–6. PMC 65005
 . PMID 11734628. doi:10.1073/pnas.261452898.
. PMID 11734628. doi:10.1073/pnas.261452898.
Further reading
- Carson SD (2002). "Receptor for the group B coxsackieviruses and adenoviruses: CAR". Reviews in Medical Virology. 11 (4): 219–26. PMID 11479928. doi:10.1002/rmv.318.
- Selinka HC, Wolde A, Sauter M, Kandolf R, Klingel K (May 2004). "Virus-receptor interactions of coxsackie B viruses and their putative influence on cardiotropism". Medical Microbiology and Immunology. 193 (2–3): 127–31. PMID 12920584. doi:10.1007/s00430-003-0193-y.
- Carson SD, Chapman NN, Tracy SM (Apr 1997). "Purification of the putative coxsackievirus B receptor from HeLa cells". Biochemical and Biophysical Research Communications. 233 (2): 325–8. PMID 9144533. doi:10.1006/bbrc.1997.6449.
- Bergelson JM, Krithivas A, Celi L, Droguett G, Horwitz MS, Wickham T, Crowell RL, Finberg RW (Jan 1998). "The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses". Journal of Virology. 72 (1): 415–9. PMC 109389
 . PMID 9420240.
. PMID 9420240. - Fechner H, Haack A, Wang H, Wang X, Eizema K, Pauschinger M, Schoemaker R, Veghel R, Houtsmuller A, Schultheiss HP, Lamers J, Poller W (Sep 1999). "Expression of coxsackie adenovirus receptor and alphav-integrin does not correlate with adenovector targeting in vivo indicating anatomical vector barriers". Gene Therapy. 6 (9): 1520–35. PMID 10490761. doi:10.1038/sj.gt.3301030.
- Bowles KR, Gibson J, Wu J, Shaffer LG, Towbin JA, Bowles NE (Oct 1999). "Genomic organization and chromosomal localization of the human Coxsackievirus B-adenovirus receptor gene". Human Genetics. 105 (4): 354–9. PMID 10543405. doi:10.1007/s004390051114.
- Bewley MC, Springer K, Zhang YB, Freimuth P, Flanagan JM (Nov 1999). "Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR". Science. 286 (5444): 1579–83. PMID 10567268. doi:10.1126/science.286.5444.1579.
- Tomko RP, Johansson CB, Totrov M, Abagyan R, Frisén J, Philipson L (Feb 2000). "Expression of the adenovirus receptor and its interaction with the fiber knob". Experimental Cell Research. 255 (1): 47–55. PMID 10666333. doi:10.1006/excr.1999.4761.
- van Raaij MJ, Chouin E, van der Zandt H, Bergelson JM, Cusack S (Nov 2000). "Dimeric structure of the coxsackievirus and adenovirus receptor D1 domain at 1.7 A resolution". Structure. 8 (11): 1147–55. PMID 11080637. doi:10.1016/S0969-2126(00)00528-1.
- Cohen CJ, Gaetz J, Ohman T, Bergelson JM (Jul 2001). "Multiple regions within the coxsackievirus and adenovirus receptor cytoplasmic domain are required for basolateral sorting". The Journal of Biological Chemistry. 276 (27): 25392–8. PMID 11316797. doi:10.1074/jbc.M009531200.
- Noutsias M, Fechner H, de Jonge H, Wang X, Dekkers D, Houtsmuller AB, Pauschinger M, Bergelson J, Warraich R, Yacoub M, Hetzer R, Lamers J, Schultheiss HP, Poller W (Jul 2001). "Human coxsackie-adenovirus receptor is colocalized with integrins alpha(v)beta(3) and alpha(v)beta(5) on the cardiomyocyte sarcolemma and upregulated in dilated cardiomyopathy: implications for cardiotropic viral infections". Circulation. 104 (3): 275–80. PMID 11457744. doi:10.1161/01.cir.104.3.275.
- Thoelen I, Magnusson C, Tågerud S, Polacek C, Lindberg M, Van Ranst M (Sep 2001). "Identification of alternative splice products encoded by the human coxsackie-adenovirus receptor gene". Biochemical and Biophysical Research Communications. 287 (1): 216–22. PMID 11549277. doi:10.1006/bbrc.2001.5535.
- He Y, Chipman PR, Howitt J, Bator CM, Whitt MA, Baker TS, Kuhn RJ, Anderson CW, Freimuth P, Rossmann MG (Oct 2001). "Interaction of coxsackievirus B3 with the full length coxsackievirus-adenovirus receptor". Nature Structural Biology. 8 (10): 874–8. PMID 11573093. doi:10.1038/nsb1001-874.
- Cohen CJ, Shieh JT, Pickles RJ, Okegawa T, Hsieh JT, Bergelson JM (Dec 2001). "The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction". Proceedings of the National Academy of Sciences of the United States of America. 98 (26): 15191–6. PMC 65005
 . PMID 11734628. doi:10.1073/pnas.261452898.
. PMID 11734628. doi:10.1073/pnas.261452898. - Law LK, Davidson BL (Jan 2002). "Adenovirus serotype 30 fiber does not mediate transduction via the coxsackie-adenovirus receptor". Journal of Virology. 76 (2): 656–61. PMC 136819
 . PMID 11752156. doi:10.1128/JVI.76.2.656-661.2002.
. PMID 11752156. doi:10.1128/JVI.76.2.656-661.2002. - van't Hof W, Crystal RG (Jun 2002). "Fatty acid modification of the coxsackievirus and adenovirus receptor". Journal of Virology. 76 (12): 6382–6. PMC 136239
 . PMID 12021372. doi:10.1128/JVI.76.12.6382-6386.2002.
. PMID 12021372. doi:10.1128/JVI.76.12.6382-6386.2002. - Walters RW, Freimuth P, Moninger TO, Ganske I, Zabner J, Welsh MJ (Sep 2002). "Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape". Cell. 110 (6): 789–99. PMID 12297051. doi:10.1016/S0092-8674(02)00912-1.