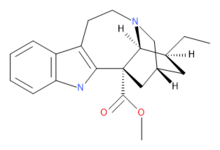
Coronaridine
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ECHA InfoCard | 100.006.727 |
| Chemical and physical data | |
| Formula | C21H26N2O2 |
| Molar mass | 338.44 g/mol |
| 3D model (JSmol) | |
| |
| |
Coronaridine, also known as 18-carbomethoxyibogamine, is an alkaloid found in Tabernanthe iboga and related species,including Tabernaemontana divaricata for which (under the now obsolete synonym Ervatamia coronaria) it was named.[1]
Coronaridine persistently reduces the self-administration of cocaine and morphine in rats.[2]
Pharmacology
Coronaridine has been reported to bind to an assortment of molecular sites, including: μ-opioid (Ki = 2.0 μM), δ-opioid (Ki = 8.1 μM), and κ-opioid receptors (Ki = 4.3 μM), NMDA receptor (Ki = 6.24 μM) (as an antagonist),[3] and nAChRs (as an antagonist).[4] It has also been found to inhibit the enzyme acetylcholinesterase, act as a voltage-gated sodium channel blocker,[5] and displays estrogenic activity in rodents.[3][4] In contrast to ibogaine and other iboga alkaloids, coronaridine does not bind to either the σ1 or σ2 receptor.[5] Coronaridine also has estrogenic properties.[3]
See also
References
- ↑ Delorenzi JC, Freire-de-Lima L, Gattass CR, et al. (July 2002). "In vitro activities of iboga alkaloid congeners coronaridine and 18-methoxycoronaridine against Leishmania amazonensis". Antimicrobial Agents and Chemotherapy. 46 (7): 2111–5. PMC 127312
 . PMID 12069962. doi:10.1128/aac.46.7.2111-2115.2002.
. PMID 12069962. doi:10.1128/aac.46.7.2111-2115.2002. - ↑ Glick SD, Kuehne ME, Raucci J, Wilson TE, Larson D, Keller RW Jr, Carlson JN (September 1994). "Effects of iboga alkaloids on morphine and cocaine self-administration in rats: relationship to tremorigenic effects and to effects on dopamine release in nucleus accumbens and striatum.". Brain Res. 657 (1-2): 14–22. PMID 7820611. doi:10.1016/0006-8993(94)90948-2.
- 1 2 3 Christophe Wiart (16 December 2013). Lead Compounds from Medicinal Plants for the Treatment of Neurodegenerative Diseases. Academic Press. pp. 67–69, 73. ISBN 978-0-12-398383-1.
- 1 2 Gideon Polya (15 May 2003). Biochemical Targets of Plant Bioactive Compounds: A Pharmacological Reference Guide to Sites of Action and Biological Effects. CRC Press. pp. 203–. ISBN 978-0-203-01371-7.
- 1 2 Chemistry and Biology. Academic Press. 21 September 1998. pp. 222–. ISBN 978-0-08-086576-8.
Treatment of drug dependence (N07B) | |
|---|---|
| Nicotine dependence | |
| Alcohol dependence | |
| Opioid dependence | |
| Benzodiazepine dependence | |
| Amphetamine dependence | |
| Cocaine dependence | |
| |||||||||||
| |||||||||||
| |||||||||||
| |||||||||||
| |||||||||||
See also: Receptor/signaling modulators | |||||||||||
| ER |
| ||||||
|---|---|---|---|---|---|---|---|
| GPER |
| ||||||
| |||||||
| Receptor (ligands) |
| ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transporter (blockers) |
| ||||||||||||||||||||||||||
| Enzyme (inhibitors) |
| ||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||
See also: Receptor/signaling modulators • GABAergics • GHBergics • Glycinergics | |||||||||||||||||||||||||||
| MOR |
|
|---|---|
| DOR |
|
| KOR |
|
| NOP |
|
| Unsorted |
|
| Others |
|
See also: Receptor/signaling modulators • Signaling peptide/protein receptor modulators | |