Carbohydrate

A carbohydrate is a biological molecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water); in other words, with the empirical formula Cm(H2O)n (where m could be different from n).[1] This formula holds true for monosaccharides. Some exceptions exist; for example, deoxyribose, a sugar component of DNA,[2] has the empirical formula C5H10O4.[3] Carbohydrates are technically hydrates of carbon;[4] structurally it is more accurate to view them as polyhydroxy aldehydes and ketones.[5]
The term is most common in biochemistry, where it is a synonym of 'saccharide', a group that includes sugars, starch, and cellulose. The saccharides are divided into four chemical groups: monosaccharides, disaccharides, oligosaccharides, and polysaccharides. Monosaccharides and disaccharides, the smallest (lower molecular weight) carbohydrates, are commonly referred to as sugars.[6] The word saccharide comes from the Greek word σάκχαρον (sákkharon), meaning "sugar". While the scientific nomenclature of carbohydrates is complex, the names of the monosaccharides and disaccharides very often end in the suffix -ose. For example, grape sugar is the monosaccharide glucose, cane sugar is the disaccharide sucrose, and milk sugar is the disaccharide lactose.
Carbohydrates perform numerous roles in living organisms. Polysaccharides serve for the storage of energy (e.g. starch and glycogen) and as structural components (e.g. cellulose in plants and chitin in arthropods). The 5-carbon monosaccharide ribose is an important component of coenzymes (e.g. ATP, FAD and NAD) and the backbone of the genetic molecule known as RNA. The related deoxyribose is a component of DNA. Saccharides and their derivatives include many other important biomolecules that play key roles in the immune system, fertilization, preventing pathogenesis, blood clotting, and development.[7]
In food science and in many informal contexts, the term carbohydrate often means any food that is particularly rich in the complex carbohydrate starch (such as cereals, bread and pasta) or simple carbohydrates, such as sugar (found in candy, jams, and desserts).
Often in lists of nutritional information, such as the USDA National Nutrient Database, the term "carbohydrate" (or "carbohydrate by difference") is used for everything other than water, protein, fat, ash, and ethanol.[8] This will include chemical compounds such as acetic or lactic acid, which are not normally considered carbohydrates. It also includes "dietary fiber" which is a carbohydrate but which does not contribute much in the way of food energy (calories), even though it is often included in the calculation of total food energy just as though it were a sugar.
Carbohydrates are found in a wide variety of foods. The important sources are cereals (wheat, maize, rice), potatoes, sugarcane, fruits, table sugar(sucrose), bread, milk, etc. Starch and sugar are the important carbohydrates in our diet. Starch is abundant in potatoes, maize, rice and other cereals. Sugar appears in our diet mainly as sucrose(table sugar) which is added to drinks and many prepared foods such as jam, biscuits and cakes. Glucose and fructose are found naturally in many fruits and some vegetables. Glycogen is carbohydrate found in the liver and muscles (as animal source). Cellulose in the cell wall of all plant tissue is a carbohydrate. It is important in our diet as fibre which helps to maintain a healthy digestive system.[9]
Structure
Formerly the name "carbohydrate" was used in chemistry for any compound with the formula Cm (H2O)n. Following this definition, some chemists considered formaldehyde (CH2O) to be the simplest carbohydrate,[10] while others claimed that title for glycolaldehyde.[11] Today, the term is generally understood in the biochemistry sense, which excludes compounds with only one or two carbons and includes many biological carbohydrates which deviate from this formula. For example, while the above representative formulas would seem to capture the commonly known carbohydrates, ubiquitous and abundant carbohydrates often deviate from this. For example, carbohydrates often display chemical groups such as: N-acetyl (e.g. chitin), sulphate (e.g. glycosaminoglycans), carboxylic acid (e.g. sialic acid) and deoxy modifications (e.g. fucose and sialic acid).
Natural saccharides are generally built of simple carbohydrates called monosaccharides with general formula (CH2O)n where n is three or more. A typical monosaccharide has the structure H–(CHOH)x(C=O)–(CHOH)y–H, that is, an aldehyde or ketone with many hydroxyl groups added, usually one on each carbon atom that is not part of the aldehyde or ketone functional group. Examples of monosaccharides are glucose, fructose, and glyceraldehydes. However, some biological substances commonly called "monosaccharides" do not conform to this formula (e.g. uronic acids and deoxy-sugars such as fucose) and there are many chemicals that do conform to this formula but are not considered to be monosaccharides (e.g. formaldehyde CH2O and inositol (CH2O)6).[12]
The open-chain form of a monosaccharide often coexists with a closed ring form where the aldehyde/ketone carbonyl group carbon (C=O) and hydroxyl group (–OH) react forming a hemiacetal with a new C–O–C bridge.
Monosaccharides can be linked together into what are called polysaccharides (or oligosaccharides) in a large variety of ways. Many carbohydrates contain one or more modified monosaccharide units that have had one or more groups replaced or removed. For example, deoxyribose, a component of DNA, is a modified version of ribose; chitin is composed of repeating units of N-acetyl glucosamine, a nitrogen-containing form of glucose.
Division
Carbohydrates are polyhydroxy aldehydes, ketones, alcohols, acids, their simple derivatives and their polymers having linkages of the acetal type. They may be classified according to their degree of polymerization and may be divided initially into three principal groups, namely sugars, oligosaccharides and polysaccharides[13]
| Class (DP*) | Subgroup | Components |
|---|---|---|
| Sugars (1–2) | Monosaccharides | Glucose, galactose, fructose, xylose |
| Disaccharides | Sucrose, lactose, maltose, trehalose | |
| Polyols | Sorbitol, mannitol | |
| Oligosaccharides (3–9) | Malto-oligosaccharides | Maltodextrins |
| Other oligosaccharides | Raffinose, stachyose, fructo-oligosaccharides | |
| Polysaccharides (>9) | Starch | Amylose, amylopectin, modified starches |
| Non-starch polysaccharides | Cellulose, hemicellulose, pectins, hydrocolloids |
DP * = Degree of polymerization
Monosaccharides

Monosaccharides are the simplest carbohydrates in that they cannot be hydrolyzed to smaller carbohydrates. They are aldehydes or ketones with two or more hydroxyl groups. The general chemical formula of an unmodified monosaccharide is (C•H2O)n, literally a "carbon hydrate". Monosaccharides are important fuel molecules as well as building blocks for nucleic acids. The smallest monosaccharides, for which n=3, are dihydroxyacetone and D- and L-glyceraldehydes.
Classification of monosaccharides
Monosaccharides are classified according to three different characteristics: the placement of its carbonyl group, the number of carbon atoms it contains, and its chiral handedness. If the carbonyl group is an aldehyde, the monosaccharide is an aldose; if the carbonyl group is a ketone, the monosaccharide is a ketose. Monosaccharides with three carbon atoms are called trioses, those with four are called tetroses, five are called pentoses, six are hexoses, and so on.[15] These two systems of classification are often combined. For example, glucose is an aldohexose (a six-carbon aldehyde), ribose is an aldopentose (a five-carbon aldehyde), and fructose is a ketohexose (a six-carbon ketone).
Each carbon atom bearing a hydroxyl group (-OH), with the exception of the first and last carbons, are asymmetric, making them stereo centers with two possible configurations each (R or S). Because of this asymmetry, a number of isomers may exist for any given monosaccharide formula. Using Le Bel-van't Hoff rule, the aldohexose D-glucose, for example, has the formula (C·H2O)6, of which four of its six carbons atoms are stereogenic, making D-glucose one of 24=16 possible stereoisomers. In the case of glyceraldehydes, an aldotriose, there is one pair of possible stereoisomers, which are enantiomers and epimers. 1, 3-dihydroxyacetone, the ketose corresponding to the aldose glyceraldehydes, is a symmetric molecule with no stereo centers. The assignment of D or L is made according to the orientation of the asymmetric carbon furthest from the carbonyl group: in a standard Fischer projection if the hydroxyl group is on the right the molecule is a D sugar, otherwise it is an L sugar. The "D-" and "L-" prefixes should not be confused with "d-" or "l-", which indicate the direction that the sugar rotates plane polarized light. This usage of "d-" and "l-" is no longer followed in carbohydrate chemistry.[16]
Ring-straight chain isomerism
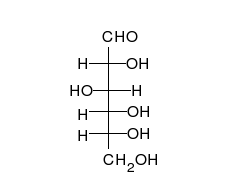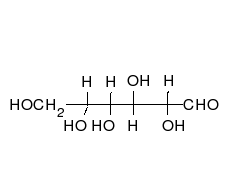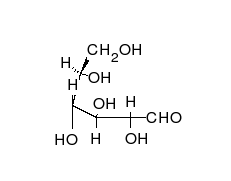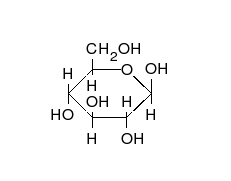
The aldehyde or ketone group of a straight-chain monosaccharide will react reversibly with a hydroxyl group on a different carbon atom to form a hemiacetal or hemiketal, forming a heterocyclic ring with an oxygen bridge between two carbon atoms. Rings with five and six atoms are called furanose and pyranose forms, respectively, and exist in equilibrium with the straight-chain form.[17]
During the conversion from straight-chain form to the cyclic form, the carbon atom containing the carbonyl oxygen, called the anomeric carbon, becomes a stereogenic center with two possible configurations: The oxygen atom may take a position either above or below the plane of the ring. The resulting possible pair of stereoisomers is called anomers. In the α anomer, the -OH substituent on the anomeric carbon rests on the opposite side (trans) of the ring from the CH2OH side branch. The alternative form, in which the CH2OH substituent and the anomeric hydroxyl are on the same side (cis) of the plane of the ring, is called the β anomer.
Use in living organisms
Monosaccharides are the major source of fuel for metabolism, being used both as an energy source (glucose being the most important in nature) and in biosynthesis. When monosaccharides are not immediately needed by many cells they are often converted to more space-efficient forms, often polysaccharides. In many animals, including humans, this storage form is glycogen, especially in liver and muscle cells. In plants, starch is used for the same purpose. The most abundant carbohydrate, cellulose, is a structural component of the cell wall of plants and many forms of algae. Ribose is a component of RNA. Deoxyribose is a component of DNA. Lyxose is a component of lyxoflavin found in the human heart.[18] Ribulose and xylulose occur in the pentose phosphate pathway. Galactose, a component of milk sugar lactose, is found in galactolipids in plant cell membranes and in glycoproteins in many tissues. Mannose occurs in human metabolism, especially in the glycosylation of certain proteins. Fructose, or fruit sugar, is found in many plants and in humans, it is metabolized in the liver, absorbed directly into the intestines during digestion, and found in semen. Trehalose, a major sugar of insects, is rapidly hydrolyzed into two glucose molecules to support continuous flight.
Disaccharides

Two joined monosaccharides are called a disaccharide and these are the simplest polysaccharides. Examples include sucrose and lactose. They are composed of two monosaccharide units bound together by a covalent bond known as a glycosidic linkage formed via a dehydration reaction, resulting in the loss of a hydrogen atom from one monosaccharide and a hydroxyl group from the other. The formula of unmodified disaccharides is C12H22O11. Although there are numerous kinds of disaccharides, a handful of disaccharides are particularly notable.
Sucrose, pictured to the right, is the most abundant disaccharide, and the main form in which carbohydrates are transported in plants. It is composed of one D-glucose molecule and one D-fructose molecule. The systematic name for sucrose, O-α-D-glucopyranosyl-(1→2)-D-fructofuranoside, indicates four things:
- Its monosaccharides: glucose and fructose
- Their ring types: glucose is a pyranose and fructose is a furanose
- How they are linked together: the oxygen on carbon number 1 (C1) of α-D-glucose is linked to the C2 of D-fructose.
- The -oside suffix indicates that the anomeric carbon of both monosaccharides participates in the glycosidic bond.
Lactose, a disaccharide composed of one D-galactose molecule and one D-glucose molecule, occurs naturally in mammalian milk. The systematic name for lactose is O-β-D-galactopyranosyl-(1→4)-D-glucopyranose. Other notable disaccharides include maltose (two D-glucoses linked α-1,4) and cellulobiose (two D-glucoses linked β-1,4). Disaccharides can be classified into two types: reducing and non-reducing disaccharides. If the functional group is present in bonding with another sugar unit, it is called a reducing disaccharide or biose.
Nutrition

Carbohydrate consumed in food yields 3.87 calories of energy per gram for simple sugars,[19] and 3.57 to 4.12 calories per gram for complex carbohydrate in most other foods.[20] Relatively high levels of carbohydrate are associated with processed foods or refined foods made from plants, including sweets, cookies and candy, table sugar, honey, soft drinks, breads and crackers, jams and fruit products, pastas and breakfast cereals. Lower amounts of carbohydrate are usually associated with unrefined foods, including beans, tubers, rice, and unrefined fruit.[21] Animal-based foods generally have the lowest carbohydrate levels, although milk does contain a high proportion of lactose.
Carbohydrates are a common source of energy in living organisms; however, no carbohydrate is an essential nutrient in humans.[22] Humans are able to obtain all of their energy requirement from protein and fats, though the potential for some negative health effects of extreme carbohydrate restriction remains, as the issue has not been studied extensively yet.[22] However, in the case of dietary fiber – indigestible carbohydrates which are not a source of energy – inadequate intake can lead to significant increases in mortality.[23]
Following a diet consisting of very low amounts of daily carbohydrate for several days will usually result in higher levels of blood ketone bodies than an isocaloric diet with similar protein content.[24] This relatively high level of ketone bodies is commonly known as ketosis and is very often confused with the potentially fatal condition often seen in type 1 diabetics known as diabetic ketoacidosis. Somebody suffering ketoacidosis will have much higher levels of blood ketone bodies along with high blood sugar, dehydration and electrolyte imbalance.
Long-chain fatty acids cannot cross the blood–brain barrier, but the liver can break these down to produce ketones. However, the medium-chain fatty acids octanoic and heptanoic acids can cross the barrier and be used by the brain, which normally relies upon glucose for its energy.[25][26][27] Gluconeogenesis allows humans to synthesize some glucose from specific amino acids: from the glycerol backbone in triglycerides and in some cases from fatty acids.
Organisms typically cannot metabolize all types of carbohydrate to yield energy. Glucose is a nearly universal and accessible source of energy. Many organisms also have the ability to metabolize other monosaccharides and disaccharides but glucose is often metabolized first. In Escherichia coli, for example, the lac operon will express enzymes for the digestion of lactose when it is present, but if both lactose and glucose are present the lac operon is repressed, resulting in the glucose being used first (see: Diauxie). Polysaccharides are also common sources of energy. Many organisms can easily break down starches into glucose; most organisms, however, cannot metabolize cellulose or other polysaccharides like chitin and arabinoxylans. These carbohydrate types can be metabolized by some bacteria and protists. Ruminants and termites, for example, use microorganisms to process cellulose. Even though these complex carbohydrates are not very digestible, they represent an important dietary element for humans, called dietary fiber. Fiber enhances digestion, among other benefits.[28]
Based on the effects on risk of heart disease and obesity in otherwise healthy middle-aged adults,[29] the Institute of Medicine recommends that American and Canadian adults get between 45–65% of dietary energy from whole-grain carbohydrates.[30] The Food and Agriculture Organization and World Health Organization jointly recommend that national dietary guidelines set a goal of 55–75% of total energy from carbohydrates, but only 10% directly from sugars (their term for simple carbohydrates).[31]
Classification
Nutritionists often refer to carbohydrates as either simple or complex. However, the exact distinction between these groups can be ambiguous. The term complex carbohydrate was first used in the U.S. Senate Select Committee on Nutrition and Human Needs publication Dietary Goals for the United States (1977) where it was intended to distinguish sugars from other carbohydrates (which were perceived to be nutritionally superior).[32] However, the report put "fruit, vegetables and whole-grains" in the complex carbohydrate column, despite the fact that these may contain sugars as well as polysaccharides. This confusion persists as today some nutritionists use the term complex carbohydrate to refer to any sort of digestible saccharide present in a whole food, where fiber, vitamins and minerals are also found (as opposed to processed carbohydrates, which provide energy but few other nutrients). The standard usage, however, is to classify carbohydrates chemically: simple if they are sugars (monosaccharides and disaccharides) and complex if they are polysaccharides (or oligosaccharides).[33]
In any case, the simple vs. complex chemical distinction has little value for determining the nutritional quality of carbohydrates.[33] Some simple carbohydrates (e.g. fructose) raise blood glucose slowly, while some complex carbohydrates (starches), especially if processed, raise blood sugar rapidly. The speed of digestion is determined by a variety of factors including which other nutrients are consumed with the carbohydrate, how the food is prepared, individual differences in metabolism, and the chemistry of the carbohydrate.[34]
The USDA's Dietary Guidelines for Americans 2010 call for moderate- to high-carbohydrate consumption from a balanced diet that includes six one-ounce servings of grain foods each day, at least half from whole grain sources and the rest from enriched.[35]
The glycemic index (GI) and glycemic load concepts have been developed to characterize food behavior during human digestion. They rank carbohydrate-rich foods based on the rapidity and magnitude of their effect on blood glucose levels. Glycemic index is a measure of how quickly food glucose is absorbed, while glycemic load is a measure of the total absorbable glucose in foods. The insulin index is a similar, more recent classification method that ranks foods based on their effects on blood insulin levels, which are caused by glucose (or starch) and some amino acids in food.
Metabolism
Carbohydrate metabolism denotes the various biochemical processes responsible for the formation, breakdown and interconversion of carbohydrates in living organisms.
The most important carbohydrate is glucose, a simple sugar (monosaccharide) that is metabolized by nearly all known organisms. Glucose and other carbohydrates are part of a wide variety of metabolic pathways across species: plants synthesize carbohydrates from carbon dioxide and water by photosynthesis storing the absorbed energy internally, often in the form of starch or lipids. Plant components are consumed by animals and fungi, and used as fuel for cellular respiration. Oxidation of one gram of carbohydrate yields approximately 4 kcal of energy, while the oxidation of one gram of lipids yields about 9 kcal. Energy obtained from metabolism (e.g., oxidation of glucose) is usually stored temporarily within cells in the form of ATP.[36] Organisms capable of aerobic respiration metabolize glucose and oxygen to release energy with carbon dioxide and water as byproducts.
Catabolism
Catabolism is the metabolic reaction which cells undergo to extract energy. There are two major metabolic pathways of monosaccharide catabolism: glycolysis and the citric acid cycle.
In glycolysis, oligo/polysaccharides are cleaved first to smaller monosaccharides by enzymes called glycoside hydrolases. The monosaccharide units can then enter into monosaccharide catabolism. In some cases, as with humans, not all carbohydrate types are usable as the digestive and metabolic enzymes necessary are not present.
Carbohydrate chemistry
Carbohydrate chemistry is a large and economically important branch of organic chemistry. Some of the main organic reactions that involve carbohydrates are:
- Carbohydrate acetalisation
- Cyanohydrin reaction
- Lobry de Bruyn–van Ekenstein transformation
- Amadori rearrangement
- Nef reaction
- Wohl degradation
- Koenigs–Knorr reaction
- Carbohydrate digestion
See also
References
- ↑ Western Kentucky University (May 29, 2013). "WKU BIO 113 Carbohydrates". wku.edu.
- ↑ Eldra Pearl Solomon; Linda R. Berg; Diana W. Martin (2004). Biology. Cengage Learning. p. 52. ISBN 978-0534278281 – via google.books.com.
- ↑ National Institute of Standards and Technology (2011). "Material Measurement Library D-erythro-Pentose, 2-deoxy-". nist.gov.
- ↑ Long Island University (May 29, 2013). "The Chemistry of Carbohydrates" (PDF). brooklyn.liu.edu.
- ↑ Purdue University (May 29, 2013). "Carbohydrates: The Monosaccharides". purdue.edu.
- ↑ Flitsch, Sabine L.; Ulijn, Rein V (2003). "Sugars tied to the spot". Nature. 421 (6920): 219–20. PMID 12529622. doi:10.1038/421219a.
- ↑ Maton, Anthea; Jean Hopkins; Charles William McLaughlin; Susan Johnson; Maryanna Quon Warner; David LaHart; Jill D. Wright (1993). Human Biology and Health. Englewood Cliffs, New Jersey, USA: Prentice Hall. pp. 52–59. ISBN 0-13-981176-1.
- ↑ USDA National Nutrient Database, 2015, p. 13
- ↑ USDA National Nutrient Database, 2015, p. 14
- ↑ John Merle Coulter, Charler Reid Barnes, Henry Chandler Cowles (1930), A Textbook of Botany for Colleges and Universities"
- ↑ Carl A. Burtis, Edward R. Ashwood, Norbert W. Tietz (2000), Tietz fundamentals of clinical chemistry
- ↑ Matthews, C. E.; K. E. Van Holde; K. G. Ahern (1999) Biochemistry. 3rd edition. Benjamin Cummings. ISBN 0-8053-3066-6
- ↑ "Carbohydrates in human nutrition - Chapter 1 - The role of carbohydrates in nutrition". Food and Agriculture Organization of the United Nations. FAO.
- ↑ Carolyn R Bertozzi. "Structural Basis of Glycan Diversity - Essentials of Glycobiology - NCBI Bookshelf". nih.gov.
- ↑ Campbell, Neil A.; Brad Williamson; Robin J. Heyden (2006). Biology: Exploring Life. Boston, Massachusetts: Pearson Prentice Hall. ISBN 0-13-250882-6.
- ↑ Pigman, Ward; Horton, D. (1972). "Chapter 1: Stereochemistry of the Monosaccharides". In Pigman and Horton. The Carbohydrates: Chemistry and Biochemistry Vol 1A (2nd ed.). San Diego: Academic Press. pp. 1–67.
- ↑ Pigman, Ward; Anet, E.F.L.J. (1972). "Chapter 4: Mutarotations and Actions of Acids and Bases". In Pigman and Horton. The Carbohydrates: Chemistry and Biochemistry Vol 1A (2nd ed.). San Diego: Academic Press. pp. 165–194.
- ↑ "lyxoflavin". Merriam-Webster.
- ↑ "Show Foods". usda.gov.
- ↑ "CALCULATION OF THE ENERGY CONTENT OF FOODS - ENERGY CONVERSION FACTORS". fao.org.
- ↑ "Carbohydrate reference list" (PDF). www.diabetes.org.uk. Retrieved October 30, 2016.
- 1 2 Westman, EC (2002). "Is dietary carbohydrate essential for human nutrition?". The American Journal of Clinical Nutrition. 75 (5): 951–3; author reply 953–4. PMID 11976176.
- ↑ Park, Y; Subar, AF; Hollenbeck, A; Schatzkin, A (2011). "Dietary fiber intake and mortality in the NIH-AARP diet and health study". Archives of Internal Medicine. 171 (12): 1061–8. PMC 3513325
 . PMID 21321288. doi:10.1001/archinternmed.2011.18.
. PMID 21321288. doi:10.1001/archinternmed.2011.18. - ↑ "Ketogenic low-carbohydrate diets have no metabolic advantage over nonketogenic low-carbohydrate diets" (PDF). nutrition.org.
- ↑ Douglas Ebert. "Energy Contribution of Octanoate to Intact Rat Brain Metabolism Measured by 13C Nuclear Magnetic Resonance Spectroscopy". jneurosci.org.
- ↑ "Heptanoate as a neural fuel: energetic and neurotransmitter precursors in normal and glucose transporter I-deficient (G1D) brain". Journal of Cerebral Blood Flow. 33: 175–182. doi:10.1038/jcbfm.2012.151.
- ↑ "Integration of Metabolism". medbio.info.
- ↑ Pichon, L; Huneau, JF; Fromentin, G; Tomé, D (2006). "A high-protein, high-fat, carbohydrate-free diet reduces energy intake, hepatic lipogenesis, and adiposity in rats". The Journal of Nutrition. 136 (5): 1256–60. PMID 16614413.
- ↑ Tighe, P; Duthie, G; Vaughan, N; Brittenden, J; Simpson, WG; Duthie, S; Mutch, W; Wahle, K; Horgan, G; Thies, F. (2010). "Effect of increased consumption of whole-grain foods on blood pressure and other cardiovascular risk markers in healthy middle-aged persons: a randomized, controlled trial". The American Journal of Clinical Nutrition. 92 (4): 733–40. PMID 20685951. doi:10.3945/ajcn.2010.29417.
- ↑ Food and Nutrition Board (2002/2005). Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. Washington, D.C.: The National Academies Press. Page 769. ISBN 0-309-08537-3.
- ↑ Joint WHO/FAO expert consultation (2003). (PDF). Geneva: World Health Organization. pp. 55–56. ISBN 92-4-120916-X.
- ↑ Joint WHO/FAO expert consultation (1998), Carbohydrates in human nutrition, chapter 1. ISBN 92-5-104114-8.
- 1 2 "Carbohydrates". The Nutrition Source. Harvard School of Public Health. Retrieved April 3, 2013.
- ↑ Jenkins, David; Alexandra L. Jenkins; Thomas M.S. Woleve; Lilian H. Thompson; A. Venkat Rao (February 1986). "Simple and Complex Carbohydrates". Nutrition Reviews. 44 (2): 44–49. doi:10.1111/j.1753-4887.1986.tb07585.x.
- ↑ DHHS and USDA, Dietary Guidelines for Americans 2010.
- ↑ Energetics of Cellular Respiration (Glucose Metabolism)
Sources
- "Compolition of foods raw, processed, prepared" (PDF). www.ars.usda.giv. September 2015. Retrieved October 30, 2016.
External links
| Wikimedia Commons has media related to Carbohydrates. |
| Wikiquote has quotations related to: Carbohydrate |
- Carbohydrates, including interactive models and animations (Requires MDL Chime)
- IUPAC-IUBMB Joint Commission on Biochemical Nomenclature (JCBN): Carbohydrate Nomenclature
- Carbohydrates detailed
- Carbohydrates and Glycosylation – The Virtual Library of Biochemistry, Molecular Biology and Cell Biology
- Functional Glycomics Gateway, a collaboration between the Consortium for Functional Glycomics and Nature Publishing Group

