Chronic wasting disease
Chronic wasting disease (CWD) is a transmissible spongiform encephalopathy (TSE) of mule deer, white-tailed deer, elk (or "wapiti"), and moose. As of 2016, CWD had only been found in members of the deer family.[1] First recognized as a clinical "wasting" syndrome in 1967 in mule deer in a wildlife research facility in northern Colorado, USA, it was identified as a TSE in 1978 and has spread to free-ranging and captive populations in 23 US states and two Canadian provinces.[2] CWD is typified by chronic weight loss leading to death. No relationship is known between CWD and any other TSE of animals or people.
Although reports in the popular press have been made of humans being affected by CWD, a study by the Centers for Disease Control and Prevention suggests, "[m]ore epidemiologic and laboratory studies are needed to monitor the possibility of such transmissions".[3] The epidemiological study further concluded, "[a]s a precaution, hunters should avoid eating deer and elk tissues known to harbor the CWD agent (e.g., brain, spinal cord, eyes, spleen, tonsils, lymph nodes) from areas where CWD has been identified".[3]
Clinical signs
Most cases of CWD occur in adult animals; the youngest animal diagnosed with natural CWD was 17 months. The disease is progressive and always fatal. The first signs are difficulties in movement. The most obvious and consistent clinical sign of CWD is weight loss over time. Behavioral changes also occur in the majority of cases, including decreased interactions with other animals, listlessness, lowering of the head, tremors, repetitive walking in set patterns, and nervousness. Excessive salivation and grinding of the teeth also are observed. Most deer show increased drinking and urination; the increased drinking and salivation may contribute to the spread of the disease.[4]
Causative agent
The agent responsible for CWD (and other TSEs, such as scrapie and bovine spongiform encephalopathy) is PRNP which is highly conserved among mammals and has been found and sequenced in deer. It is a prion, an abnormal form of a normal protein, known as prion protein (PrP), most commonly found in the central nervous system (CNS), and is capable of spreading to the peripheral nervous system (PNS), thus infecting meat, or muscle, of deer and elk. The abnormal PrP infects the host animal by promoting conversion of normal cellular prion protein (PrPC) to the abnormal prion form (PrPres or PrPd). The build-up of PrPd in the brain is associated with widespread neurodegeneration.[4][5][6]
Diagnosis
Diagnosis is based on post mortem examination (necropsy) and testing; examination of the dead body is not definitive as many animals die early in the course of the disease and conditions found are non-specific; general signs of poor health and Aspiration pneumonia, which may be the actual cause of death, are common. On microscopic examination, lesions of CWD in the central nervous system resemble those of other TSEs. In addition, scientists use immunohistochemistry to test brain, lymph, and neuroendocrine tissues for the presence of the abnormal prion protein to diagnose CWD; positive IHC findings in the obex is considered the gold standard.[4]
As of 2015 there were no commercially feasible diagnostic tests that could be used on live animals. It is possible to run a bioassay, taking fluids from cervids suspected of infection and incubating them in transgenic mice that express the cervid prion protein, to determine if the cervid is infected, but there are ethical issues with this and it is not scalable.[4]
Epidemiology
The origin and mode of transmission of the prions causing CWD is unknown, but recent research indicates that prions can be excreted by deer and elk, and are transmitted by eating grass growing in contaminated soil.[7][8] Animals born in captivity and those born in the wild have been affected with the disease. Based on epidemiology, transmission of CWD is thought to be lateral (from animal to animal). Maternal transmission may occur, although it appears to be relatively unimportant in maintaining epidemics. An infected deer's saliva is able to spread the CWD prions.[9] Exposure between animals is associated with sharing food and water sources contaminated with CWD prions shed by diseased deer.[10]
The disease was first identified in 1967 in a closed herd of captive mule deer in contiguous portions of northeastern Colorado. In 1980, the disease was determined to be a TSE. It was first identified in wild elk and mules in 1981 in Colorado and Wyoming, and in farmed elk in 1997.[4][5][6]
In May 2001, CWD was also found in free-ranging deer in the southwestern corner of Nebraska (adjacent to Colorado and Wyoming) and later in additional areas in western Nebraska. The limited area of northern Colorado, southern Wyoming, and western Nebraska in which free-ranging deer, moose, and/or elk positive for CWD have been found is referred to as the endemic area. The area in 2006 has expanded to six states, including parts of eastern Utah, southwestern South Dakota, and northwestern Kansas. Also, areas not contiguous (to the endemic area) areas in central Utah and central Nebraska have been found. The limits of the affected areas are not well defined, since the disease is at a low incidence and the amount of sampling may not be adequate to detect it. In 2002, CWD was detected in wild deer in south-central Wisconsin and northern Illinois and in an isolated area of southern New Mexico. In 2005, it was found in wild white-tailed deer in New York and in Hampshire County, West Virginia.[11] In 2008, the first confirmed case of CWD in Michigan was discovered in an infected deer on an enclosed deer-breeding facility. It is also found in the Canadian provinces of Alberta and Saskatchewan.
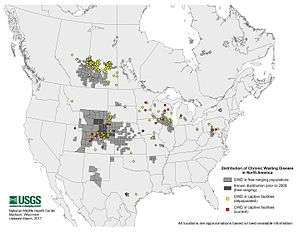
In February 2011, the Maryland Department of Natural Resources reported the first confirmed case of the disease in that state. The affected animal was a white-tailed deer killed by a hunter.[12]
CWD has also been diagnosed in farmed elk and deer herds in a number of states and in two Canadian provinces. The first positive farmed elk herd in the United States was detected in 1997 in South Dakota. Since then, additional positive elk herds and farmed white-tailed deer herds have been found in South Dakota (7), Nebraska (4), Colorado (10), Oklahoma (1), Kansas (1), Minnesota (3), Montana (1), Wisconsin (6) and New York (2). As of fall of 2006, four positive elk herds in Colorado and a positive white-tailed deer herd in Wisconsin remain under state quarantine. All of the other herds have been depopulated or have been slaughtered and tested, and the quarantine has been lifted from one herd that underwent rigorous surveillance with no further evidence of disease. CWD also has been found in farmed elk in the Canadian provinces of Saskatchewan and Alberta. A retrospective study also showed mule deer exported from Denver to the Toronto Zoo in the 1980s were affected. In June 2015, the disease was detected in a male white-tailed deer on a breeding ranch in Medina County, Texas. State officials euthanized 34 deer in an effort to contain a possible outbreak.
Species that have been affected with CWD include elk, mule deer, white-tailed deer, black-tailed deer, and moose. Other ruminant species, including wild ruminants and domestic cattle, sheep, and goats, have been housed in wildlife facilities in direct or indirect contact with CWD-affected deer and elk, with no evidence of disease transmission. However, experimental transmission of CWD into other ruminants by intracranial inoculation does result in disease, suggesting only a weak molecular species barrier exists. Research is ongoing to further explore the possibility of transmission of CWD to other species.
By April 2016 CWD had been found in captive animals in South Korea; the disease arrived there with live elk that were imported for farming in the late 1990s.[1]
Europe
In 2016, the first case of CWD in Europe was from the Nordfjella free ranging reindeer in Southern Norway. Scientists surveyed the diseased female reindeer until the reindeer died and used the carcass to isolate the prions. The main origin of CWD to Norway is still unknown, whereas importation of infected deer was the contamination source in South Korea. Norway has strict legislation and rules not allowing importation of live animals and cervids into the country. Norway has had a scrapie surveillance program since 1997; while no reports of scrapie within the range of Nordfjella reindeer sup population have been identified, sheep are herded through that region and are a potential source of infection.[13]
In each of May and June, infected wild moose were found around 300 km north from the first case, in Selbu.[14] By the end of August, a fourth case had been confirmed in a wild reindeer shot in the same area as the first case in March.[15]
Transmission pathways
Direct transmission
CWD may be directly transmitted via contact with infected animals, their bodily tissues, and their bodily fluids.[16] Transmission may result from contact with both clinically affected and infected, but asymptomatic, cervids.[17]
Recent research on Rocky Mountain elk found that with CWD-infected dams, many sub-clinical, there was a high rate (80%) of maternal-to-offspring transmission of CWD prions, regardless of gestational period.[17] While not dispositive relative to disease development in the fetus, this does suggest that maternal transmission may be yet another important route of direct CWD transmission.
Experimental transmission
In addition to the cervid species in which CWD is known to naturally occur, Black-tailed deer and European red deer have been clinically demonstrated to be naturally susceptible to CWD.[18] Other cervid species, including reindeer and caribou, are also suspected to be naturally vulnerable to this disease.[16] Many other non-cervid mammalian species have been experimentally infected with CWD, either orally or via intracerebral inoculation.[16] These species include monkeys, sheep, cattle, prairie voles, mice, and ferrets.[19]
An experimental case study of oral transmission of CWD to reindeer shows certain reindeer breeds may be susceptible to CWD while other sub-populations may be protective against CWD in free ranging populations. None of the reindeer in the study showed symptoms of CWD potentially signifying resistance to different CWD strains.[20]
Indirect/environmental transmission
Environmental transmission has been linked to contact with infected bodily fluids and tissues, as well as contact with contaminated environments. Once in the environment, CWD prions may remain infectious for many years. Thus, decomposition of diseased carcasses, infected "gut piles" from hunters who field dress their cervid harvests, as well as the urine, saliva, feces, and antler velvet of infected individuals that are deposited in the environment, all have the potential to create infectious environmental reservoirs of CWD.[4]
One avian scavenger, the American crow, was recently evaluated as a potential vector for CWD.[21] As CWD prions remain viable after passing through the bird's digestive tract, crows represent a possible mechanism for the creation of environmental reservoirs of CWD.[21][22] Additionally, the crows' extensive geographic range presents ample opportunities for them to come in contact with CWD. This coupled with the population density and longevity of communal roosting sites in both urban and rural locations suggests that the fecal deposits at roosting sites may represent a CWD environmental reservoir.[21] Conservative estimates for crows' fecal deposits at one winter roosting site for one winter season ranged from 391,552 - 599,032 kg.[21]
CWD prions adhere so tightly to soil surface particles that the ground itself becomes a source of infection and may be a major route of transmission due to frequent ground contact when cervids graze.[4]
The potential for human exposure to CWD
As of 2013 there was no evidence of transmission to humans from cervids, nor by eating cervids, but both channels remain a subject of public health surveillance and research.[6]
Research
Research is focused on better ways to monitor disease in the wild, live animal diagnostic tests, developing vaccines, better ways to dispose of animals who died from the disease and to decontaminate the environment, where prions can persist in soils, and better ways to monitor the food supply.[6]
References
| Wikimedia Commons has media related to Chronic wasting disease. |
- 1 2 Rachel Becker for Nature News. April 18, 2016 Deadly animal prion disease appears in Europe
- ↑ "Chronic Wasting Disease". USGS National Wildlife Health Center. 2013-05-21. Retrieved 2014-12-05.
- 1 2 Belay, E.D.; Maddox, R.A.; Williams, E.S.; Miller, M.W.; Gambetti, P.; Schonberger, L.B. (June 2004). "Chronic Wasting Disease and Potential Transmission to Humans". Emerging Infectious Diseases. CDC. 10 (6): 977–984. PMC 3323184
 . PMID 15207045. doi:10.3201/eid0905.020577. Retrieved 2008-04-08.
. PMID 15207045. doi:10.3201/eid0905.020577. Retrieved 2008-04-08. - 1 2 3 4 5 6 7 Haley NJ, Hoover EA. Chronic wasting disease of cervids: current knowledge and future perspectives. Annu Rev Anim Biosci. 2015;3:305-25. Review. 25387112
- 1 2 USGS National Wildlife Health Center Frequently asked questions concerning Chronic Wasting Disease (CWD) Page last updated May 21, 2013; page accessed April 25, 2016
- 1 2 3 4 Patrice N Klein, CWD Program Manager USDA /APHIS. Chronic Wasting Disease WHHCC Meeting – 5–6 February 2013
- ↑ "Study Shows Prions Stick Around In Certain Soils". Science Daily. September 17, 2003. Retrieved October 23, 2006.
- ↑ "New Research Supports Theory That Indirect Transmission Of Chronic Wasting Disease Possible In Mule Deer". Science Daily. May 19, 2004. Retrieved October 23, 2006.
- ↑ Mathiason CK, Powers JG, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mason GL, Hays SA, Hayes-Klug J, Seelig DM, Wild MA, Wolfe LL, Spraker TR, Miller MW, Sigurdson CJ, Telling GC, Hoover EA (October 6, 2006). "Infectious prions in the saliva and blood of deer with chronic wasting disease". Science. 314 (5796): 133–6. PMID 17023660. doi:10.1126/science.1132661.
- ↑ Ernest, Holly B.; Hoar, Bruce R.; Well, Jay A.; O'Rourke, Katherine I. (2010-04-01). "Molecular genealogy tools for white-tailed deer with chronic wasting disease". Canadian Journal of Veterinary Research = Revue Canadienne De Recherche Veterinaire. 74 (2): 153–156. ISSN 1928-9022. PMC 2851727
 . PMID 20592847.
. PMID 20592847. - ↑ "Chronic Wasting Disease". West Virginia Division of Natural Resources. 2008-08-01.
- ↑ "Wasting Disease Confirmed in State". February 13, 2011.
- ↑ Benestad, Sylvie L.; Mitchell, Gordon; Simmons, Marion; Ytrehus, Bjørnar; Vikøren, Turid (2016-01-01). "First case of chronic wasting disease in Europe in a Norwegian free-ranging reindeer". Veterinary Research. 47. ISSN 0928-4249. PMC 5024462
 . PMID 27641251. doi:10.1186/s13567-016-0375-4.
. PMID 27641251. doi:10.1186/s13567-016-0375-4. - ↑ Norwegian Food Safety Authority. Chronic Wasting Disease in Norway. Published July 13, 2016; updated July 14, 2016
- ↑ The Norwegian veterinary Institute. Nytt tilfelle av CWD på villrein
- 1 2 3 Saunders, S.E.; Bartelt-Hunt, S.L.; Bartz, J.C. (2012). "Occurrence, transmission, and zoonotic potential of chronic wasting disease". Emerging Infectious Diseases. 18 (3): 369–376. PMC 3309570
 . PMID 22377159. doi:10.3201/eid1803.110685.
. PMID 22377159. doi:10.3201/eid1803.110685. - 1 2 Selariu, A.; Powers, J.G.; Nalls, A.; Brandhuber, M.; Mayfield, A...&; Mathiason, C.K. (2015). "In utero transmission and tissue distribution of chronic wasting disease-associated prions in free-ranging Rocky Mountain elk". Journal of General Virology.
- ↑ Williams, E.S.; Young, S. (1980). "Chronic wasting disease of captive mule deer: A Spongiform Encepalopathy". Journal of Wildlife Diseases. 16 (1): 89–98. doi:10.7589/0090-3558-16.1.89.
- ↑ Wisniewski, T.; Goni, F. (2012). "Could immodulation be used to prevent prion diseases?". Expert Review of Anti-Infective Therapy. 10 (3): 307–317. doi:10.1586/eri.11.177.
- ↑ Mitchell, Gordon B.; Sigurdson, Christina J.; O’Rourke, Katherine I.; Algire, James; Harrington, Noel P.; Walther, Ines; Spraker, Terry R.; Balachandran, Aru (2012-06-18). "Experimental Oral Transmission of Chronic Wasting Disease to Reindeer (Rangifer tarandus tarandus)". PLoS ONE. 7 (6): e39055. ISSN 1932-6203. PMC 3377593
 . PMID 22723928. doi:10.1371/journal.pone.0039055.
. PMID 22723928. doi:10.1371/journal.pone.0039055. - 1 2 3 4 Fischer, J.W.; Phillips, G.E.; Nichols, T.A.; Vercauteren, K.C. (2013). "Could avian scavengers translocated infectious prions to disease-free areas initiating new foci of chronic wasting disease?". Prion. 7 (4): 263–266. doi:10.4161/pri.25621.
- ↑ Vercauteren, K.C.; Pilon, J.L.; Nash, P.B.; Phillips, G.E.; Fischer, J.W. (2012). "Prion remains infectious after passage through digestive system of American crows (Corvus brachyrhynchos)". PLOS ONE. 7 (10): e45774. PMC 3474818
 . PMID 23082115. doi:10.1371/journal.pone.0045774.
. PMID 23082115. doi:10.1371/journal.pone.0045774.
External links
- This entry incorporates public domain text originally from the Animal and Plant Health Inspection Service, APHIS.
- Chronic Wasting Disease Alliance
- "Chronic Wasting Disease (CWD) of Deer and Elk". Canadian Food Inspection Agency. 2006-10-03. Archived from the original on September 25, 2006. Retrieved October 23, 2006.
- Elk Research Institute
- United States Geological Survey
- United States Department of Agriculture
- Colorado Division of Wildlife
- Illinois Department of Natural Resources
- Minnesota Department of Natural Resources
- Nebraska Game and Parks Commission
- New York State Department of Environmental Conservation
- Wisconsin Department of Natural Resources
- Wyoming Game and Fish
- Wildlife Disease Information Node Chronic Wasting Disease Factsheet and resources
- A case of chronic wasting disease in a captive red deer (Cervus elaphus)