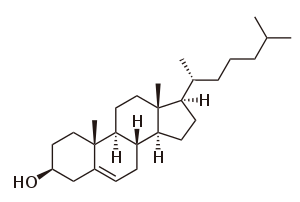
Cholesterol
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(3β)-cholest-5-en-3-ol | |
| Systematic IUPAC name
2,15-dimethyl-14-(1,5-dimethylhexyl)tetracyclo[8.7.0.02,7.011,15]heptadec-7-en-5-ol | |
| Other names
(10R,13R)-10,13-dimethyl-17-(6-methylheptan-2-yl)-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol, Cholesterin, Cholesteryl alcohol[1] | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.000.321 |
| KEGG | |
| PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C27H46O | |
| Molar mass | 386.65 g/mol |
| Appearance | white crystalline powder[2] |
| Density | 1.052 g/cm3 |
| Melting point | 148 to 150 °C (298 to 302 °F; 421 to 423 K) [2] |
| Boiling point | 360 °C (680 °F; 633 K) (decomposes) |
| 1.8 mg/L (30 °C)[3] | |
| Solubility | soluble in acetone, benzene, chloroform, ethanol, ether, hexane, isopropyl myristate, methanol |
| -284.2·10−6 cm3/mol | |
| Hazards | |
| Flash point | 209.3 ±12.4 °C [1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Cholesterol, from the Ancient Greek chole- (bile) and stereos (solid) followed by the chemical suffix -ol for an alcohol, is an organic molecule. It is a sterol (or modified steroid),[4] a type of lipid molecule, and is biosynthesized by all animal cells, because it is an essential structural component of all animal cell membranes; essential to maintain both membrane structural integrity and fluidity. Cholesterol enables animal cells to dispense with a cell wall (to protect membrane integrity and cell viability), thereby allowing animal cells to change shape rapidly and animals to move (unlike bacteria and plant cells, which are restricted by their cell walls).
In addition to its importance for animal cell structure, cholesterol also serves as a precursor for the biosynthesis of steroid hormones, bile acid,[5] and vitamin D. Cholesterol is the principal sterol synthesized by all animals. In vertebrates, hepatic cells typically produce the greatest amounts. It is absent among prokaryotes (bacteria and archaea), although there are some exceptions, such as Mycoplasma, which require cholesterol for growth.[6]
François Poulletier de la Salle first identified cholesterol in solid form in gallstones in 1769. However, it was not until 1815 that chemist Michel Eugène Chevreul named the compound "cholesterine".[7][8]
Physiology
Since cholesterol is essential for all animal life, each cell is capable of synthesizing it by way of a complex 37-step process, beginning with the mevalonate pathway and ending with a 19-step conversion of lanosterol to cholesterol. Furthermore, it can be absorbed directly from animal-based foods.
A human male weighing 68 kg (150 lb) normally synthesizes about 1 gram (1,000 mg) per day, and his body contains about 35 g, mostly contained within the cell membranes. Typical daily cholesterol dietary intake for a man in the United States is 307 mg (above the upper limit recommended by the Dietary Guidelines Advisory Committee[9]).
Most ingested cholesterol is esterified, and esterified cholesterol is poorly absorbed. The body also compensates for any absorption of additional cholesterol by reducing cholesterol synthesis.[10] For these reasons, cholesterol in food, seven to ten hours after ingestion, has little, if any effect on concentrations of cholesterol in the blood. However, during the first seven hours after ingestion of cholesterol, as absorbed fats are being distributed around the body within extracellular water by the various lipoproteins (which transport all fats in the water outside cells), the concentrations increase.[11] It is also important to recognize, however, that the concentrations measured in the samples of blood plasma vary with the measurement methods used. Traditional, cheaper methods do not reflect (a) which lipoproteins are transporting the various fat molecules, nor (b) which cells are ingesting, burning or exporting the fat molecules being measured as totals from samples of blood plasma.
Cholesterol is recycled in the body. The liver excretes it in a non-esterified form (via bile) into the digestive tract. Typically, about 50% of the excreted cholesterol is reabsorbed by the small intestine back into the bloodstream.
Plants make cholesterol in very small amounts.[12] Plants manufacture phytosterols (substances chemically similar to cholesterol), which can compete with cholesterol for reabsorption in the intestinal tract, thus potentially reducing cholesterol reabsorption.[13] When intestinal lining cells absorb phytosterols, in place of cholesterol, they usually excrete the phytosterol molecules back into the GI tract, an important protective mechanism.
Function
Cholesterol, given that it composes about 30% of all animal cell membranes, is required to build and maintain membranes and modulates membrane fluidity over the range of physiological temperatures. The hydroxyl group on cholesterol interacts with the polar heads of the membrane phospholipids and sphingolipids, while the bulky steroid and the hydrocarbon chain are embedded in the membrane, alongside the nonpolar fatty-acid chain of the other lipids. Through the interaction with the phospholipid fatty-acid chains, cholesterol increases membrane packing, which both alters membrane fluidity[14] and maintains membrane integrity so that animal cells do not need to build cell walls (like plants and most bacteria). The membrane remains stable and durable without being rigid, allowing animal cells to change shape and animals to move.
The structure of the tetracyclic ring of cholesterol contributes to the fluidity of the cell membrane, as the molecule is in a trans conformation making all but the side chain of cholesterol rigid and planar.[15] In this structural role, cholesterol also reduces the permeability of the plasma membrane to neutral solutes,[16] hydrogen ions, and sodium ions.[17]
Within the cell membrane, cholesterol also functions in intracellular transport, cell signaling and nerve conduction. Cholesterol is essential for the structure and function of invaginated caveolae and clathrin-coated pits, including caveola-dependent and clathrin-dependent endocytosis. The role of cholesterol in endocytosis of these types can be investigated by using methyl beta cyclodextrin (MβCD) to remove cholesterol from the plasma membrane. Recent studies show that cholesterol is also implicated in cell signaling processes, assisting in the formation of lipid rafts in the plasma membrane, which brings receptor proteins in close proximity with high concentrations of second messenger molecules.[18] In multiple layers, cholesterol and phospholipids, both electrical insulators, can facilitate speed of transmission of electrical impulses along nerve tissue. For many neuron fibers, a myelin sheath, rich in cholesterol since it is derived from compacted layers of Schwann cell membrane, provides insulation for more efficient conduction of impulses.[19] Demyelination (loss of some of these Schwann cells) is believed to be part of the basis for multiple sclerosis.
Within cells, cholesterol is also a precursor molecule for several biochemical pathways. For example, it is the precursor molecule for the synthesis of vitamin D and all steroid hormones, including the adrenal gland hormones cortisol and aldosterone, as well as the sex hormones progesterone, estrogens, and testosterone, and their derivatives.[5][20]
The liver excretes cholesterol into biliary fluids, which is then stored in the gallbladder. Bile contains bile salts, which solubilize fats in the digestive tract and aid in the intestinal absorption of fat molecules as well as the fat-soluble vitamins, A, D, E, and K.
Biosynthesis
All animal cells manufacture cholesterol, for both membrane structure and other uses, with relative production rates varying by cell type and organ function. About 20% of total daily cholesterol production occurs in the liver; other sites of higher synthesis rates include the intestines, adrenal glands, and reproductive organs.
Synthesis within the body starts with the mevalonate pathway where two molecules of acetyl CoA condense to form acetoacetyl-CoA. This is followed by a second condensation between acetyl CoA and acetoacetyl-CoA to form 3-hydroxy-3-methylglutaryl CoA (HMG-CoA).[21]

This molecule is then reduced to mevalonate by the enzyme HMG-CoA reductase. Production of mevalonate is the rate-limiting and irreversible step in cholesterol synthesis and is the site of action for statins (a class of cholesterol lowering drugs).

Mevalonate is finally converted to isopentenyl pyrophosphate (IPP) through two phosphorylation steps and one decarboxylation step that requires ATP.

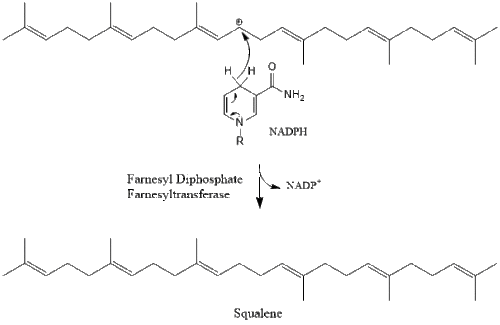
Three molecules of isopentenyl pyrophosphate condense to form farnesyl pyrophosphate through the action of geranyl transferase.


Two molecules of farnesyl pyrophosphate then condense to form squalene by the action of squalene synthase in the endoplasmic reticulum.[21]

Oxidosqualene cyclase then cyclizes squalene to form lanosterol. Finally, lanosterol is converted to cholesterol through a 19-step process.[22][23]



The final 19 steps to cholesterol contain NADPH and Oxygen to help oxidize methyl groups for removal of carbons, mutases to move alkene groups, and NADH to help reduce ketones.


Konrad Bloch and Feodor Lynen shared the Nobel Prize in Physiology or Medicine in 1964 for their discoveries concerning some of the mechanisms and methods of regulation of cholesterol and fatty acid metabolism, though lots of complexities remain to be sorted out.
Regulation of cholesterol synthesis
Biosynthesis of cholesterol is directly regulated by the cholesterol levels present, though the homeostatic mechanisms involved are only partly understood. A higher intake from food leads to a net decrease in endogenous production, whereas lower intake from food has the opposite effect. The main regulatory mechanism is the sensing of intracellular cholesterol in the endoplasmic reticulum by the protein SREBP (sterol regulatory element-binding protein 1 and 2).[24] In the presence of cholesterol, SREBP is bound to two other proteins: SCAP (SREBP cleavage-activating protein) and INSIG-1. When cholesterol levels fall, INSIG-1 dissociates from the SREBP-SCAP complex, which allows the complex to migrate to the Golgi apparatus. Here SREBP is cleaved by S1P and S2P (site-1 protease and site-2 protease), two enzymes that are activated by SCAP when cholesterol levels are low.
The cleaved SREBP then migrates to the nucleus, and acts as a transcription factor to bind to the sterol regulatory element (SRE), which stimulates the transcription of many genes. Among these are the low-density lipoprotein (LDL) receptor and HMG-CoA reductase. The LDL receptor scavenges circulating LDL from the bloodstream, whereas HMG-CoA reductase leads to an increase of endogenous production of cholesterol.[25] A large part of this signaling pathway was clarified by Dr. Michael S. Brown and Dr. Joseph L. Goldstein in the 1970s. In 1985, they received the Nobel Prize in Physiology or Medicine for their work. Their subsequent work shows how the SREBP pathway regulates expression of many genes that control lipid formation and metabolism and body fuel allocation.
Cholesterol synthesis can also be turned off when cholesterol levels are high. HMG-CoA reductase contains both a cytosolic domain (responsible for its catalytic function) and a membrane domain. The membrane domain senses signals for its degradation. Increasing concentrations of cholesterol (and other sterols) cause a change in this domain's oligomerization state, which makes it more susceptible to destruction by the proteosome. This enzyme's activity can also be reduced by phosphorylation by an AMP-activated protein kinase. Because this kinase is activated by AMP, which is produced when ATP is hydrolyzed, it follows that cholesterol synthesis is halted when ATP levels are low.[26]
Dietary sources
Animal fats are complex mixtures of triglycerides (stored energy, see: "Introduction to Energy Storage".), with lesser amounts of both the phospholipids and cholesterol molecules from which all animal (and human) cell membranes are constructed. Since all animal cells manufacture cholesterol, all animal-based foods contain cholesterol in varying amounts.[27] Major dietary sources of cholesterol include cheese, egg yolks, beef, pork, poultry, fish, and shrimp.[28] Human breast milk also contains significant quantities of cholesterol.[29]
From a dietary perspective, plant cells do not manufacture cholesterol, and it is not found in plant foods.[28][30] Some plant foods, such as avocado, flax seeds and peanuts, contain phytosterols, which compete with cholesterol for absorption in the intestines, reducing the absorption of both dietary and bile cholesterol.[31] However, a typical diet contributes on the order of 0.2 grams of phytosterols, which is not enough to have a significant impact on blocking cholesterol absorption. Phytosterols intake can be supplemented through the use of phytosterol-containing functional foods or dietary supplements that are recognized as having potential to reduce levels of LDL-cholesterol.[32] Some supplemental guidelines have recommended doses of phytosterols in the 1.6-3.0 grams per day range (Health Canada, EFSA, ATP III, FDA). A recent meta-analysis demonstrating a 12% reduction in LDL-cholesterol at a mean dose of 2.1 grams per day.[33] However, the benefits of a diet supplemented with phytosterols have been questioned.[34]
In 2016, the United States Department of Agriculture Dietary Guidelines Advisory Committee recommended that Americans eat as little dietary cholesterol as possible.[35] Increased dietary intake of industrial trans fats is associated with an increased risk in all-cause mortality and cardiovascular diseases.[36] Trans fats have been shown to reduce levels of HDL while increasing levels of LDL.[37] Based on such evidence and evidence implicating low HDL and high LDL levels in cardiovascular disease (see Hypercholesterolemia), many health authorities advocate reducing LDL-cholesterol through changes in diet in addition to other lifestyle modifications.[38] Rats subjected to high-fat or fructose diets became dyslipidemic.[39] However, well designed, adequately powered randomized controlled trials investigating patient-relevant outcomes of low-fat diets for otherwise healthy people with hypercholesterolaemia are lacking. Moreover, for familial hypercholesterolaemia, large, parallel, randomized controlled trials are still needed to investigate the effectiveness of a cholesterol-lowering diet and the addition of omega-3 fatty acids, soya protein, plant sterols or stanols.[40][41]
Plasma transport and regulation of absorption

As an isolated molecule, cholesterol is only minimally soluble in water; it dissolves into the (water-based) bloodstream only at exceedingly small concentrations. Instead, cholesterol is transported within lipoproteins, complex discoidal particles with exterior amphiphilic proteins and lipids, whose outward-facing surfaces are water-soluble and inward-facing surfaces are lipid-soluble; i.e. transport via emulsification. Triglycerides and cholesterol esters are carried internally. Phospholipids and cholesterol, being amphipathic, are transported in the monolayer surface of the lipoprotein particle.
There are several types of lipoproteins in the blood. In order of increasing density, they are chylomicrons, very-low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), low-density lipoprotein (LDL), and high-density lipoprotein (HDL). Lower protein/lipid ratios make for less dense lipoproteins. Cholesterol within different lipoproteins is identical, although some is carried as its native "free" alcohol form (the cholesterol-OH group facing the water surrounding the particles), while others as fatty acyl esters, known also as cholesterol esters, within the particles.
Lipoprotein particles are organized by complex apolipoproteins, typically 80-100 different proteins per particle, which can be recognized and bound by specific receptors on cell membranes, directing their lipid payload into specific cells and tissues currently ingesting these fat transport particles. Lipoprotein particles thus include a molecular addresses which play key roles in distribution and delivery of fats around the body in the water outside cells.
Chylomicrons, the least dense cholesterol transport molecules, contain apolipoprotein B-48, apolipoprotein C, and apolipoprotein E (the principal cholesterol carrier in the brain[42]) in their shells. Chylomicrons carry fats from the intestine to muscle and other tissues in need of fatty acids for energy or fat production. Unused cholesterol remains in more cholesterol-rich chylomicron remnants, and taken up from here to the bloodstream by the liver.
VLDL molecules are produced by the liver from triacylglycerol and cholesterol which was not used in the synthesis of bile acids. These molecules contain apolipoprotein B100 and apolipoprotein E in their shells, and are degraded by lipoprotein lipase on the blood vessel wall to IDL.
Blood vessels cleave and absorb triacylglycerol from IDL molecules, increasing the concentration of cholesterol. IDL molecules are then consumed in two processes: half is metabolized by HTGL and taken up by the LDL receptor on the liver cell surfaces, while the other half continues to lose triacylglycerols in the bloodstream until they become LDL molecules, with the highest concentration of cholesterol within them.
LDL particles are the major blood cholesterol carriers. Each one contains approximately 1,500 molecules of cholesterol ester. LDL molecule shells contain just one molecule of apolipoprotein B100, recognized by LDL receptors in peripheral tissues. Upon binding of apolipoprotein B100, many LDL receptors concentrate in clathrin-coated pits. Both LDL and its receptor form vesicles within a cell via endocytosis. These vesicles then fuse with a lysosome, where the lysosomal acid lipase enzyme hydrolyzes the cholesterol esters. The cholesterol can then be used for membrane biosynthesis or esterified and stored within the cell, so as to not interfere with the cell membranes.
LDL receptors are used up during cholesterol absorption, and its synthesis is regulated by SREBP, the same protein that controls the synthesis of cholesterol de novo, according to its presence inside the cell. A cell with abundant cholesterol will have its LDL receptor synthesis blocked, to prevent new cholesterol in LDL molecules from being taken up. Conversely, LDL receptor synthesis proceeds when a cell is deficient in cholesterol.
When this process becomes unregulated, LDL molecules without receptors begin to appear in the blood. These LDL molecules are oxidized and taken up by macrophages, which become engorged and form foam cells. These foam cells often become trapped in the walls of blood vessels and contribute to atherosclerotic plaque formation. Differences in cholesterol homeostasis affect the development of early atherosclerosis (carotid intima-media thickness).[43] These plaques are the main causes of heart attacks, strokes, and other serious medical problems, leading to the association of so-called LDL cholesterol (actually a lipoprotein) with "bad" cholesterol.[26]
HDL particles are thought to transport cholesterol back to the liver, either for excretion or for other tissues that synthesize hormones, in a process known as reverse cholesterol transport (RCT).[44] Large numbers of HDL particles correlates with better health outcomes.,[45] whereas low numbers of HDL particles is associated with atheromatous disease progression in the arteries.
Metabolism, recycling and excretion
Cholesterol is susceptible to oxidation and easily forms oxygenated derivatives known as oxysterols. Three different mechanisms can form these: autoxidation, secondary oxidation to lipid peroxidation, and cholesterol-metabolizing enzyme oxidation. A great interest in oxysterols arose when they were shown to exert inhibitory actions on cholesterol biosynthesis.[46] This finding became known as the “oxysterol hypothesis”. Additional roles for oxysterols in human physiology include their participation in bile acid biosynthesis, function as transport forms of cholesterol, and regulation of gene transcription.[47]
In biochemical experiments radiolabelled forms of cholesterol, such as tritiated-cholesterol are used. These derivatives undergo degradation upon storage and it is essential to purify cholesterol prior to use. Cholesterol can be purified using small Sephadex LH-20 columns.[48]
Cholesterol is oxidized by the liver into a variety of bile acids.[49] These, in turn, are conjugated with glycine, taurine, glucuronic acid, or sulfate. A mixture of conjugated and nonconjugated bile acids, along with cholesterol itself, is excreted from the liver into the bile. Approximately 95% of the bile acids are reabsorbed from the intestines, and the remainder are lost in the feces.[50] The excretion and reabsorption of bile acids forms the basis of the enterohepatic circulation, which is essential for the digestion and absorption of dietary fats. Under certain circumstances, when more concentrated, as in the gallbladder, cholesterol crystallises and is the major constituent of most gallstones (lecithin and bilirubin gallstones also occur, but less frequently).[51] Every day, up to 1 g of cholesterol enters the colon. This cholesterol originates from the diet, bile, and desquamated intestinal cells, and can be metabolized by the colonic bacteria. Cholesterol is converted mainly into coprostanol, a nonabsorbable sterol that is excreted in the feces. A cholesterol-reducing bacterium origin has been isolated from human feces.[52]
Although cholesterol is a steroid generally associated with mammals, the human pathogen Mycobacterium tuberculosis is able to completely degrade this molecule and contains a large number of genes that are regulated by its presence.[53] Many of these cholesterol-regulated genes are homologues of fatty acid β-oxidation genes, but have evolved in such a way as to bind large steroid substrates like cholesterol.[54][55]
Research
Cholesterol binds to and affects the gating of a number of ion channels such as the nicotinic acetylcholine receptor, GABAA receptor, and the inward-rectifier potassium ion channel.[56] Cholesterol also activates the estrogen-related receptor alpha (ERRα), and may be the endogenous ligand for the receptor.[57][58] The constitutively active nature of the receptor may be explained by the fact that cholesterol is ubiquitous in the body.[58] Inhibition of ERRα signaling by reduction of cholesterol production has been identified as a key mediator of the effects of statins and bisphosphonates on bone, muscle, and macrophages.[57][58] On the basis of these findings, it has been suggested that the ERRα should be de-orphanized and classified as a receptor for cholesterol.[57][58]
Clinical significance
Hypercholesterolemia

According to the lipid hypothesis, since cholesterol (like all fat molecules) is transported around the body (in the water outside cells) inside lipoprotein particles, elevated cholesterol concentrations (hypercholesterolemia) — potentially offers a lower cost way to estimate concentrations of LDL particles; possibly even low concentrations of functional HDL particles — both variations strongly associated with cardiovascular disease because LDL particles promote atheroma development in arteries (atherosclerosis).
This atherosclerotic disease process, over decades, leads to myocardial infarction (heart attack), stroke, and peripheral vascular disease. Since higher blood LDL, especially higher LDL particle concentrations and smaller LDL particle size, contribute to this process more than the cholesterol content of the HDL particles,[59] LDL particles are often termed "bad cholesterol" because they have been linked to atheroma formation. On the other hand, high concentrations of functional HDL, which can remove cholesterol from cells and atheroma, offer protection and are sometimes referred to as "good cholesterol". These balances are mostly genetically determined, but can be changed by body build, medications, food choices,[60] and other factors.[61]
Conditions with elevated concentrations of oxidized LDL particles, especially "small dense LDL" (sdLDL) particles, are associated with atheroma formation in the walls of arteries, a condition known as atherosclerosis, which is the principal cause of coronary heart disease and other forms of cardiovascular disease. In contrast, HDL particles (especially large HDL) have been identified as a mechanism by which cholesterol and inflammatory mediators can be removed from atheroma. Increased concentrations of HDL correlate with lower rates of atheroma progressions and even regression. A 2007 study pooling data on almost 900,000 subjects in 61 cohorts demonstrated that blood total cholesterol levels have an exponential effect on cardiovascular and total mortality, with the association more pronounced in younger subjects. Still, because cardiovascular disease is relatively rare in the younger population, the impact of high cholesterol on health is still larger in older people.[62]
Elevated levels of the lipoprotein fractions, LDL, IDL and VLDL are regarded as atherogenic (prone to cause atherosclerosis).[63] Levels of these fractions, rather than the total cholesterol level, correlate with the extent and progress of atherosclerosis. Conversely, the total cholesterol can be within normal limits, yet be made up primarily of small LDL and small HDL particles, under which conditions atheroma growth rates would still be high. Recently, a post hoc analysis of the IDEAL and the EPIC prospective studies found an association between high levels of HDL cholesterol (adjusted for apolipoprotein A-I and apolipoprotein B) and increased risk of cardiovascular disease, casting doubt on the cardioprotective role of "good cholesterol".[64][65]
Elevated cholesterol levels are treated with a strict diet consisting of low saturated fat, trans fat-free, low cholesterol foods,[66][67] often followed by one of various hypolipidemic agents, such as statins, fibrates, cholesterol absorption inhibitors, nicotinic acid derivatives or bile acid sequestrants.[68] Extreme cases have previously been treated with partial ileal bypass surgery, which has now been superseded by medication. Apheresis-based treatments are still used for very severe hyperlipidemias that are either unresponsive to treatment or require rapid lowering of blood lipids.[69] There are several international guidelines on the treatment of hypercholesterolaemia.[70]
Multiple human trials using HMG-CoA reductase inhibitors, known as statins, have repeatedly confirmed that changing lipoprotein transport patterns from unhealthy to healthier patterns significantly lowers cardiovascular disease event rates, even for people with cholesterol values currently considered low for adults.[71] Studies have also found that statins reduce atheroma progression.[72] As a result, people with a history of cardiovascular disease may derive benefit from statins irrespective of their cholesterol levels (total cholesterol below 5.0 mmol/L [193 mg/dL]),[73] and in men without cardiovascular disease, there is benefit from lowering abnormally high cholesterol levels ("primary prevention").[74] Primary prevention in women was originally practiced only by extension of the findings in studies on men,[75] since, in women, none of the large statin trials conducted prior to 2007 demonstrated a statistically significant reduction in overall mortality or in cardiovascular endpoints.[76] In 2008, a large clinical trial reported that, in apparently healthy adults with increased levels of the inflammatory biomarker high-sensitivity C-reactive protein but with low initial LDL, 20 mg/day of rosuvastatin for 1.9 years resulted in a 44% reduction in the incidence of cardiovascular events and a 20% reduction in all-cause mortality; the effect was statistically significant for both genders.[77] Though this result was met with some skepticism, later studies and meta-analyses likewise demonstrated statistically significant (but smaller) reductions in all-cause and cardiovascular mortality, without significant heterogeneity by gender.[78]
| Level mg/dL | Level mmol/L | Interpretation |
| < 200 | < 5.2 | Desirable level corresponding to lower risk for heart disease |
| 200–240 | 5.2–6.2 | Borderline high risk |
| > 240 | > 6.2 | High risk |
The 1987 report of National Cholesterol Education Program, Adult Treatment Panels suggests the total blood cholesterol level should be: < 200 mg/dL normal blood cholesterol, 200–239 mg/dL borderline-high, > 240 mg/dL high cholesterol.[79] The American Heart Association provides a similar set of guidelines for total (fasting) blood cholesterol levels and risk for heart disease:[80]
However, as today's testing methods determine LDL ("bad") and HDL ("good") cholesterol separately, this simplistic view has become somewhat outdated. The desirable LDL level is considered to be less than 130 mg/dL (2.6 mmol/L),[81] although a newer upper limit of 70 mg/dL (1.8 mmol/L) can be considered in higher-risk individuals based on some of the above-mentioned trials. A ratio of total cholesterol to HDL—another useful measure—of far less than 5:1 is thought to be healthier.

Total cholesterol is defined as the sum of HDL, LDL, and VLDL. Usually, only the total, HDL, and triglycerides are measured. For cost reasons, the VLDL is usually estimated as one-fifth of the triglycerides and the LDL is estimated using the Friedewald formula (or a variant): estimated LDL = [total cholesterol] − [total HDL] − [estimated VLDL]. VLDL can be calculated by dividing total triglycerides by five. Direct LDL measures are used when triglycerides exceed 400 mg/dL. The estimated VLDL and LDL have more error when triglycerides are above 400 mg/dL.[82]
Given the well-recognized role of cholesterol in cardiovascular disease, some studies have shown an inverse correlation between cholesterol levels and mortality. A 2009 study of patients with acute coronary syndromes found an association of hypercholesterolemia with better mortality outcomes.[83] In the Framingham Heart Study, in subjects over 50 years of age, they found an 11% increase overall and 14% increase in cardiovascular disease mortality per 1 mg/dL per year drop in total cholesterol levels. The researchers attributed this phenomenon to the fact that people with severe chronic diseases or cancer tend to have below-normal cholesterol levels.[84] This explanation is not supported by the Vorarlberg Health Monitoring and Promotion Programme, in which men of all ages and women over 50 with very low cholesterol were likely to die of cancer, liver diseases, and mental diseases. This result indicates the low-cholesterol effect occurs even among younger respondents, contradicting the previous assessment among cohorts of older people that this is a proxy or marker for frailty occurring with age.[85]
Although the vast majority of doctors and medical scientists consider that there is a link between cholesterol and atherosclerosis as discussed above,[86] a 2014 meta-analysis of over 500,000 patients concluded there is insufficient evidence to support the recommendation of high consumption of polyunsaturated fatty acids and low consumption of total saturated fats for cardiovascular health.[87]
Hypocholesterolemia
Abnormally low levels of cholesterol are termed hypocholesterolemia. Research into the causes of this state is relatively limited, but some studies suggest a link with depression, cancer, and cerebral hemorrhage. In general, the low cholesterol levels seem to be a consequence, rather than a cause, of an underlying illness.[62] A genetic defect in cholesterol synthesis causes Smith-Lemli-Opitz syndrome, which is often associated with low plasma cholesterol levels. Hyperthyroidism, or any other endocrine disturbance which causes upregulation of the LDL receptor, may result in hypocholesterolemia.[88]
Cholesterol testing
The American Heart Association recommends testing cholesterol every 4–6 years for people aged 20 years or older.[89] A separate set of American Heart Association guidelines issued in 2013 indicates that patients taking statin medications should have their cholesterol tested 4–12 weeks after their first dose and then every 3–12 months thereafter.[90]
A blood sample after 12-hour fasting is taken by a doctor, or a home cholesterol-monitoring device is used to determine a lipoprotein profile. This measures total cholesterol, LDL (bad) cholesterol, HDL (good) cholesterol, and triglycerides. It is recommended to test cholesterol at least every five years if a person has total cholesterol of 5.2 mmol/L or more (200+ mg/dL), or if a man over age 45 or a woman over age 50 has HDL (good) cholesterol less than 1 mmol/L (40 mg/dL), or there are other risk factors for heart disease and stroke. Other risk factors for heart disease include Diabetes, Hypertension (or use of anti-hypertensive medications), low HDL, family history of CAD and hypercholesterolemia, and cigarette smoking.[91]
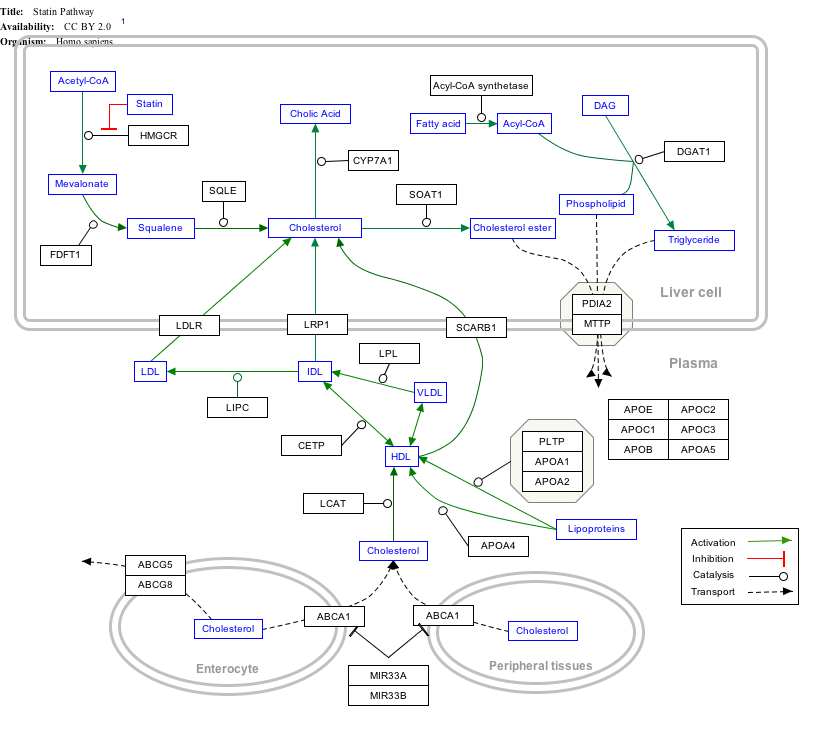
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
|px|alt=Statin Pathway edit]]
Statin Pathway edit
- ↑ The interactive pathway map can be edited at WikiPathways: "Statin_Pathway_WP430".
Cholesteric liquid crystals
Some cholesterol derivatives (among other simple cholesteric lipids) are known to generate the liquid crystalline "cholesteric phase". The cholesteric phase is, in fact, a chiral nematic phase, and it changes colour when its temperature changes. This makes cholesterol derivatives useful for indicating temperature in liquid crystal display thermometers and in temperature-sensitive paints.
Stereoisomers
Cholesterol has 256 stereoisomers that arise from its 8 stereocenters, although only two of the stereoisomers are of biochemical significance (nat-cholesterol and ent-cholesterol, for natural and enantiomer, respectively),[92][93] and only one occurs naturally (nat-cholesterol).

See also
- Arcus senilis "Cholesterol ring" in the eyes
- Cardiovascular disease
- Cholesterol embolism
- Cholesterol total synthesis
- Familial hypercholesterolemia
- List of cholesterol in foods
- Niemann–Pick disease Type C
- Oxycholesterol
- Remnant cholesterol
Additional images
 Cholesterol units conversion
Cholesterol units conversion Steroidogenesis, using cholesterol as building material
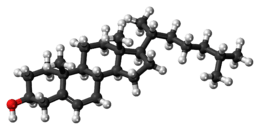
Steroidogenesis, using cholesterol as building material Space-filling model of the Cholesterol molecule
Space-filling model of the Cholesterol molecule Numbering of the steroid nuclei
Numbering of the steroid nuclei
References
- 1 2 "Substance Data for 57-88-5".
- 1 2 "Safety (MSDS) data for cholesterol". Retrieved 2007-10-20.
- ↑ http://www.pnas.org/content/70/8/2313.full.pdf
- ↑ Cholesterol at the US National Library of Medicine Medical Subject Headings (MeSH)
- 1 2 Hanukoglu I (Dec 1992). "Steroidogenic enzymes: structure, function, and role in regulation of steroid hormone biosynthesis.". J Steroid Biochem Mol Biol. 43 (8): 779–804. PMID 22217824. doi:10.1016/0960-0760(92)90307-5.
- ↑ Razin S, Tully JG (May 1970). "Cholesterol Requirement of Mycoplasmas". Journal of Bacteriology. 102 (2): 306–310. PMC 247552
 . PMID 4911537.
. PMID 4911537. - ↑ Chevreul (1816) "Recherches chimiques sur les corps gras, et particulièrement sur leurs combinaisons avec les alcalis. Sixième mémoire. Examen des graisses d'homme, de mouton, de boeuf, de jaguar et d'oie" (Chemical researches on fatty substances, and particularly on their combinations o filippos ine kapios with alkalis. Sixth memoir. Study of human, sheep, beef, jaguar and goose fat), Annales de Chimie et de Physique, 2 : 339-372. From page 346 : "Je nommerai cholesterine, de χολη, bile, et στερεος, solide, la substance cristallisée des calculs biliares humains, ... " (I will name cholesterine — from χολη (bile) and στερεος (solid) — the crystalized substance from human gallstones ... )
- ↑ Olson RE (February 1998). "Discovery of the lipoproteins, their role in fat transport and their significance as risk factors". J. Nutr. 128 (2 Suppl): 439S–443S. PMID 9478044.
- ↑ "National Health and Nutrition Examination Survey" (PDF). United States Center for Disease Control. Retrieved 2012-01-28.
- ↑ Lecerf JM, de Lorgeril M (2011). "Dietary cholesterol: from physiology to cardiovascular risk". Br J Nutr. 106 (1): 6–14. PMID 21385506. doi:10.1017/S0007114511000237.
- ↑ Dubois C, Armand M, Mekki N, Portugal H, Pauli AM, Bernard PM, Lafont H, Lairon D (1994). "Effects of increasing amounts of dietary cholesterol on postprandial lipemia and lipoproteins in human subjects". Journal of LIPID Research. 35 (1994): 1993–2007. PMID 7868978.
- ↑ Behrman EJ, Gopalan V (2005). Scovell WM, ed. "Cholesterol and Plants" (PDF). Journal of Chemical Education. 82 (12): 1791. Bibcode:2005JChEd..82.1791B. doi:10.1021/ed082p1791.
- ↑ John S, Sorokin AV, Thompson PD (February 2007). "Phytosterols and vascular disease". Curr. Opin. Lipidol. 18 (1): 35–40. PMID 17218830. doi:10.1097/MOL.0b013e328011e9e3.
- ↑ Sadava D, Hillis DM, Heller HC, Berenbaum MR (2011). Life: The Science of Biology 9th Edition. San Francisco: Freeman. pp. 105–114. ISBN 1-4292-4646-4.
- ↑ Ohvo-Rekilä H, Ramstedt B, Leppimäki P, Slotte JP (January 2002). "Cholesterol interactions with phospholipids in membranes". Prog. Lipid Res. 41 (1): 66–97. PMID 11694269. doi:10.1016/S0163-7827(01)00020-0.
- ↑ Yeagle PL (October 1991). "Modulation of membrane function by cholesterol". Biochimie. 73 (10): 1303–10. PMID 1664240. doi:10.1016/0300-9084(91)90093-G.
- ↑ Haines TH (July 2001). "Do sterols reduce proton and sodium leaks through lipid bilayers?". Prog. Lipid Res. 40 (4): 299–324. PMID 11412894. doi:10.1016/S0163-7827(01)00009-1.
- ↑ Incardona JP, Eaton S (April 2000). "Cholesterol in signal transduction". Curr. Opin. Cell Biol. 12 (2): 193–203. PMID 10712926. doi:10.1016/S0955-0674(99)00076-9.
- ↑ Pawlina W, Ross MW (2006). Histology: a text and atlas: with correlated cell and molecular biology. Philadelphia: Lippincott Wiliams & Wilkins. p. 230. ISBN 0-7817-5056-3.
- ↑ Payne AH, Hales DB (2004). "Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones". Endocrine Reviews. 25 (6): 947–70. PMID 15583024. doi:10.1210/er.2003-0030.
- 1 2 "Biosynthesis and Regulation of Cholesterol (with Animation)". PharmaXChange.info.
- ↑ Berg J (2002). Biochemistry. New York: WH Freeman. ISBN 0-7167-3051-0.
- ↑ Rhodes CM, Stryer L, Tasker R (1995). Biochemistry (4th ed.). San Francisco: W.H. Freeman. pp. 280, 703. ISBN 0-7167-2009-4.
- ↑ Espenshade PJ, Hughes AL (2007). "Regulation of sterol synthesis in eukaryotes". Annu. Rev. Genet. 41: 401–27. PMID 17666007. doi:10.1146/annurev.genet.41.110306.130315.
- ↑ Brown MS, Goldstein JL (1997). "The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor". Cell. 89 (3): 331–40. PMID 9150132. doi:10.1016/S0092-8674(00)80213-5.
- 1 2 Tymoczko JL, Berg T, Stryer L, Berg JM (2002). Biochemistry. San Francisco: W.H. Freeman. pp. 726–727. ISBN 0-7167-4955-6.
- ↑ William W C (2003). Lipid analysis: isolation, separation, identification, and structural analysis of lipids. Ayr, Scotland: Oily Press. ISBN 0-9531949-5-7.
- 1 2 "USDA National Nutrient Database for Standard Reference, Release 21" (PDF). United States Department of Agriculture. Retrieved 2008-10-24.
- ↑ Jensen RG, Hagerty MM, McMahon KE (1 June 1978). "Lipids of human milk and infant formulas: a review" (PDF). Am J Clin Nutr. 31 (6): 990–1016. PMID 352132.
- ↑ Behrman EJ, Gopalan V (December 2005). "Cholesterol and Plants". J. Chem. Educ. 82 (12): 1791. Bibcode:2005JChEd..82.1791B. doi:10.1021/ed082p1791.
- ↑ De Smet, E; Mensink, R. P.; Plat, J (2012). "Effects of plant sterols and stanols on intestinal cholesterol metabolism: Suggested mechanisms from past to present". Molecular Nutrition & Food Research. 56 (7): 1058–72. PMID 22623436. doi:10.1002/mnfr.201100722.
- ↑ European Food Safety Authority, Journal (2010). "Scientific opinion on the substantiation of health claims related to plant sterols and plant stanols and maintenance of normal blood cholesterol concentrations".
- ↑ Ras RT, Geleijnse JM, Trautwein EA (2014). "LDL-cholesterol-lowering effect of plant sterols and stanols across different dose ranges: a meta-analysis of randomised controlled studies". Br. J. Nutr. 112 (2): 214–9. PMC 4071994
 . PMID 24780090. doi:10.1017/S0007114514000750.
. PMID 24780090. doi:10.1017/S0007114514000750. - ↑ Weingärtner O, Böhm M, Laufs U (February 2009). "Controversial role of plant sterol esters in the management of hypercholesterolaemia". Eur. Heart J. 30 (4): 404–9. PMC 2642922
 . PMID 19158117. doi:10.1093/eurheartj/ehn580.
. PMID 19158117. doi:10.1093/eurheartj/ehn580. - ↑ "2015-2020 Dietary Guidelines: Answers to Your Questions. What are "eating patterns" and why does the 2015-2020 Dietary Guidelines focus on them?". ChooseMyPlate.gov, US Department of Agriculture. January 2016. Retrieved 17 February 2017.
- ↑ de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, Uleryk E, Budylowski P, Schünemann H, Beyene J, Anand SS (August 2015). "Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies". British Medical Association. 351: h3978. PMC 4532752
 . PMID 26268692. doi:10.1136/bmj.h3978.
. PMID 26268692. doi:10.1136/bmj.h3978. - ↑ Ascherio A, Willett WC (October 1997). "Health effects of trans fatty acids". Am. J. Clin. Nutr. 66 (4 Suppl): 1006S–1010S. PMID 9322581.
- ↑ "High cholesterol levels by NHS". National Health Service. Retrieved 2010-09-14.
- ↑ Balakumar, M.; Raji, L.; Prabhu, D.; Sathishkumar, C.; Prabu, P.; Mohan, V.; Balasubramanyam, M. (2016-12-01). "High-fructose diet is as detrimental as high-fat diet in the induction of insulin resistance and diabetes mediated by hepatic/pancreatic endoplasmic reticulum (ER) stress". Molecular and Cellular Biochemistry. 423 (1-2): 93–104. ISSN 1573-4919. PMID 27699590. doi:10.1007/s11010-016-2828-5.
- ↑ Smart, Neil A.; Marshall, Belinda J.; Daley, Maxine; Boulos, Elie; Windus, Janelle; Baker, Nadine; Kwok, Nigel (2011-02-16). "Low-fat diets for acquired hypercholesterolaemia". The Cochrane Database of Systematic Reviews (2): CD007957. ISSN 1469-493X. PMID 21328303. doi:10.1002/14651858.CD007957.pub2.
- ↑ Shafiq, Nusrat; Singh, Meenu; Kaur, Sharonjeet; Khosla, Pratibha; Malhotra, Samir (2010-01-20). "Dietary treatment for familial hypercholesterolaemia". The Cochrane Database of Systematic Reviews (1): CD001918. ISSN 1469-493X. PMID 20091526. doi:10.1002/14651858.CD001918.pub2.
- ↑ Mahley, R. W. (2016). "Apolipoprotein E: From cardiovascular disease to neurodegenerative disorders". Journal of Molecular Medicine. 94 (7): 739–46. PMC 4921111
 . PMID 27277824. doi:10.1007/s00109-016-1427-y.
. PMID 27277824. doi:10.1007/s00109-016-1427-y. - ↑ Weingärtner O, Pinsdorf T, Rogacev KS, Blömer L, Grenner Y, Gräber S, Ulrich C, Girndt M, Böhm M, Fliser D, Laufs U, Lütjohann D, Heine GH (2010). Federici M, ed. "The relationships of markers of cholesterol homeostasis with carotid intima-media thickness". PLoS ONE. 5 (10): e13467. Bibcode:2010PLoSO...513467W. PMC 2956704
 . PMID 20976107. doi:10.1371/journal.pone.0013467.
. PMID 20976107. doi:10.1371/journal.pone.0013467. - ↑ Lewis GF, Rader DJ (June 2005). "New insights into the regulation of HDL metabolism and reverse cholesterol transport". Circ. Res. 96 (12): 1221–32. PMID 15976321. doi:10.1161/01.RES.0000170946.56981.5c.
- ↑ Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR, Bangdiwala S, Tyroler HA (January 1989). "High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies". Circulation. 79 (1): 8–15. PMID 2642759. doi:10.1161/01.CIR.79.1.8.
- ↑ Kandutsch AA, Chen HW, Heiniger HJ (August 1978). "Biological activity of some oxygenated sterols". Science. 201 (4355): 498–501. Bibcode:1978Sci...201..498K. PMID 663671. doi:10.1126/science.663671.
- ↑ Russell DW (December 2000). "Oxysterol biosynthetic enzymes". Biochim. Biophys. Acta. 1529 (1–3): 126–35. PMID 11111082. doi:10.1016/S1388-1981(00)00142-6.
- ↑ Hanukoglu I, Jefcoate CR (1980). "Pregnenolone separation from cholesterol using Sephadex LH-20 mini-columns". Journal of Chromatography A. 190 (1): 256–262. doi:10.1016/S0021-9673(00)85545-4.
- ↑ Javitt NB (December 1994). "Bile acid synthesis from cholesterol: regulatory and auxiliary pathways". FASEB J. 8 (15): 1308–11. PMID 8001744.
- ↑ Wolkoff AW, Cohen DE (February 2003). "Bile acid regulation of hepatic physiology: I. Hepatocyte transport of bile acids". Am. J. Physiol. Gastrointest. Liver Physiol. 284 (2): G175–9. PMID 12529265. doi:10.1152/ajpgi.00409.2002.
- ↑ Marschall HU, Einarsson C (June 2007). "Gallstone disease". J. Intern. Med. 261 (6): 529–42. PMID 17547709. doi:10.1111/j.1365-2796.2007.01783.x.
- ↑ Gérard P, Lepercq P, Leclerc M, Gavini F, Raibaud P, Juste C (September 2007). "Bacteroides sp. strain D8, the first cholesterol-reducing bacterium isolated from human feces". Appl. Environ. Microbiol. 73 (18): 5742–9. PMC 2074900
 . PMID 17616613. doi:10.1128/AEM.02806-06.
. PMID 17616613. doi:10.1128/AEM.02806-06. - ↑ Wipperman MF, Sampson NS, Thomas ST (2014). "Pathogen roid rage: Cholesterol utilization by Mycobacterium tuberculosis". Crit. Rev. Biochem. Mol. Biol. 49 (4): 269–93. PMC 4255906
 . PMID 24611808. doi:10.3109/10409238.2014.895700.
. PMID 24611808. doi:10.3109/10409238.2014.895700. - ↑ Thomas ST, Sampson NS (2013). "Mycobacterium tuberculosis utilizes a unique heterotetrameric structure for dehydrogenation of the cholesterol side chain". Biochemistry. 52 (17): 2895–2904. PMC 3726044
 . PMID 23560677. doi:10.1021/bi4002979.
. PMID 23560677. doi:10.1021/bi4002979. - ↑ Wipperman MF, Yang M, Thomas ST, Sampson NS (2013). "Shrinking the FadE Proteome of Mycobacterium tuberculosis: Insights into Cholesterol Metabolism through Identification of an α2β2 Heterotetrameric Acyl Coenzyme A Dehydrogenase Family". J. Bacteriol. 195 (19): 4331–4341. PMC 3807453
 . PMID 23836861. doi:10.1128/JB.00502-13.
. PMID 23836861. doi:10.1128/JB.00502-13. - ↑ Levitan I, Singh DK, Rosenhouse-Dantsker A (2014). "Cholesterol binding to ion channels". Frontiers in Physiology. 5: 65. PMC 3935357
 . PMID 24616704. doi:10.3389/fphys.2014.00065.
. PMID 24616704. doi:10.3389/fphys.2014.00065. - 1 2 3 Wei W, Schwaid AG, Wang X, Wang X, Chen S, Chu Q, Saghatelian A, Wan Y (March 2016). "Ligand Activation of ERRα by Cholesterol Mediates Statin and Bisphosphonate Effects". Cell Metabolism. 23 (3): 479–91. PMC 4785078
 . PMID 26777690. doi:10.1016/j.cmet.2015.12.010.
. PMID 26777690. doi:10.1016/j.cmet.2015.12.010. - 1 2 3 4 Nuclear Receptors in Development and Disease. Elsevier Science. 17 May 2017. pp. 88–. ISBN 978-0-12-802196-5.
- ↑ Brunzell JD, Davidson M, Furberg CD, Goldberg RB, Howard BV, Stein JH, Witztum JL (April 2008). "Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation". Diabetes Care. 31 (4): 811–22. PMID 18375431. doi:10.2337/dc08-9018.
- ↑ Department of Health (UK), NHS Choices, "More evidence for Mediterranean diet". 8 March 2011. Access date: Nov 11, 2015
- ↑ Durrington P (August 2003). "Dyslipidaemia". Lancet. 362 (9385): 717–31. PMID 12957096. doi:10.1016/S0140-6736(03)14234-1.
- 1 2 Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R, Collins R (December 2007). "Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths". Lancet. 370 (9602): 1829–39. PMID 18061058. doi:10.1016/S0140-6736(07)61778-4.
- ↑ "Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report" (PDF). National Institutes of Health. National Heart, Lung and Blood Institute. Retrieved 2008-10-27.
- ↑ van der Steeg WA, Holme I, Boekholdt SM, Larsen ML, Lindahl C, Stroes ES, Tikkanen MJ, Wareham NJ, Faergeman O, Olsson AG, Pedersen TR, Khaw KT, Kastelein JJ (February 2008). "High-density lipoprotein cholesterol, high-density lipoprotein particle size, and apolipoprotein A-I: significance for cardiovascular risk: the IDEAL and EPIC-Norfolk studies". J. Am. Coll. Cardiol. 51 (6): 634–42. PMID 18261682. doi:10.1016/j.jacc.2007.09.060.
- ↑ Robinson JG, Wang S, Jacobson TA (2012). "Meta-analysis of comparison of effectiveness of lowering apolipoprotein B versus low-density lipoprotein cholesterol and nonhigh-density lipoprotein cholesterol for cardiovascular risk reduction in randomized trials". The American Journal of Cardiology. 110 (10): 1468–76. PMID 22906895. doi:10.1016/j.amjcard.2012.07.007.
- ↑ "How Can I Lower High Cholesterol" (PDF). American Heart Association. Retrieved 2011-04-03.
- ↑ "Diseases and Conditions. High cholesterol: Olive oil, Foods with added plant sterols or stanols, Other changes to your diet". Mayo Clinic. 2012. Retrieved 11 November 2015.
- ↑ National Institute for Health and Clinical Excellence. Clinical guideline 67: Lipid modification. London, 2008.
- ↑ Matthew Lui, Ross Garberich, Craig Strauss, Thomas Davin, and Thomas Knickelbine, Usefulness of Lipid Apheresis in the Treatment of Familial Hypercholesterolemia Journal of Lipids, vol. 2014, Article ID 864317, 6 pages, 2014. doi:10.1155/2014/864317
- ↑ Mannu GS, Zaman MJ, Gupta A, Rehman HU, Myint PK (2012). "Update on guidelines for management of hypercholesterolemia". Expert Review of Cardiovascular Therapy. 10 (10): 1239–49. PMID 23190064. doi:10.1586/erc.12.94.
- ↑ Kizer JR, Madias C, Wilner B, Vaughan CJ, Mushlin AI, Trushin P, Gotto AM, Pasternak RC (May 1, 2010). "Relation of different measures of low-density lipoprotein cholesterol to risk of coronary artery disease and death in a meta-regression analysis of large-scale trials of statin therapy.". The American journal of cardiology. 105 (9): 1289–96. PMC 2917836
 . PMID 20403481. doi:10.1016/j.amjcard.2009.12.051.
. PMID 20403481. doi:10.1016/j.amjcard.2009.12.051. - ↑ Nicholls SJ (August 2008). "Rosuvastatin and progression of atherosclerosis". Expert Rev Cardiovasc Ther. 6 (7): 925–33. PMID 18666843. doi:10.1586/14779072.6.7.925.
- ↑ Heart Protection Study Collaborative Group. (July 2002). "MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial". Lancet. 360 (9326): 7–22. PMID 12114036. doi:10.1016/S0140-6736(02)09327-3.
- ↑ Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ (November 1995). "Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group". N. Engl. J. Med. 333 (20): 1301–7. PMID 7566020. doi:10.1056/NEJM199511163332001.
- ↑ Grundy SM (May 2007). "Should women be offered cholesterol lowering drugs to prevent cardiovascular disease? Yes". BMJ. 334 (7601): 982–982. PMC 1867899
 . PMID 17494017. doi:10.1136/bmj.39202.399942.AD.
. PMID 17494017. doi:10.1136/bmj.39202.399942.AD. - ↑ Kendrick M (May 2007). "Should women be offered cholesterol lowering drugs to prevent cardiovascular disease? No". BMJ. 334 (7601): 983–983. PMC 1867901
 . PMID 17494018. doi:10.1136/bmj.39202.397488.AD.
. PMID 17494018. doi:10.1136/bmj.39202.397488.AD. - ↑ JUPITER Study Group (2008). "Rosuvastatin to Prevent Vascular Events in Men and Women with Elevated C-Reactive Protein". N Engl J Med. 359 (21): 2195–2207. PMID 18997196. doi:10.1056/nejmoa0807646.
- ↑ Brugts JJ, Yetgin T, Hoeks SE, Gotto AM, Shepherd J, Westendorp RG, de Craen AJ, Knopp RH, Nakamura H, Ridker P, van Domburg R, Deckers JW (2009). "The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials". BMJ. 338: b2376. PMC 2714690
 . PMID 19567909. doi:10.1136/bmj.b2376.
. PMID 19567909. doi:10.1136/bmj.b2376. - ↑ "Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. The Expert Panel". Arch. Intern. Med. 148 (1): 36–69. January 1988. PMID 3422148. doi:10.1001/archinte.148.1.36.
- ↑ "Cholesterol". American Heart Association. 17 November 2008. Retrieved 2009-02-21.
- ↑ "About cholesterol". American Heart Association. Archived from the original on 20 October 2001.
- ↑ Warnick GR, Knopp RH, Fitzpatrick V, Branson L (January 1990). "Estimating low-density lipoprotein cholesterol by the Friedewald equation is adequate for classifying patients on the basis of nationally recommended cutpoints". Clin. Chem. 36 (1): 15–9. PMID 2297909.
- ↑ Wang TY, Newby LK, Chen AY, Mulgund J, Roe MT, Sonel AF, Bhatt DL, DeLong ER, Ohman EM, Gibler WB, Peterson ED (September 2009). "Hypercholesterolemia paradox in relation to mortality in acute coronary syndrome". Clin Cardiol. 32 (9): E22–8. PMID 19645040. doi:10.1002/clc.20518.
- ↑ Anderson KM, Castelli WP, Levy D (April 1987). "Cholesterol and mortality. 30 years of follow-up from the Framingham study". JAMA. 257 (16): 2176–80. PMID 3560398. doi:10.1001/jama.257.16.2176.
- ↑ Ulmer H, Kelleher C, Diem G, Concin H (2004). "Why Eve is not Adam: prospective follow-up in 149650 women and men of cholesterol and other risk factors related to cardiovascular and all-cause mortality". J Women's Health (Larchmt). 13 (1): 41–53. PMID 15006277. doi:10.1089/154099904322836447.
- ↑ Sternberg D (2007). The Cholesterol Wars: The Cholesterol Skeptics vs the Preponderance of Evidence. Boston: Academic Press. ISBN 0-12-373979-9.
- ↑ Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, Franco OH, Butterworth AS, Forouhi NG, Thompson SG, Khaw KT, Mozaffarian D, Danesh J, Di Angelantonio E (2014). "Association of dietary, circulating, and supplement fatty acids with coronary risk: A systematic review and meta-analysis". Annals of Internal Medicine. 160 (6): 398–406. PMID 24723079. doi:10.7326/M13-1788.
- ↑ Rizos CV, Elisaf MS, Liberopoulos EN (24 February 2011). "Effects of thyroid dysfunction on lipid profile". The Open Cardiovascular Medicine Journal. 5 (1): 76––84. PMC 3109527
 . PMID 21660244. doi:10.2174/1874192401105010076.
. PMID 21660244. doi:10.2174/1874192401105010076. - ↑ "How To Get Your Cholesterol Tested". American Heart Association. Retrieved 2013-07-10.
- ↑ Stone NJ, Robinson J, Goff DC (2013). "Getting a grasp of the Guidelines". American College of Cardiology. Retrieved 2 April 2014.
- ↑ "Implications of Recent Clinical Trials for the ATP III Guidelines". National Heart, Lungs and Blood Institute. Archived from the original on 2 February 2014. Retrieved 2014-01-27.
- ↑ Westover EJ, Covey DF, Brockman HL, Brown RE, Pike LJ (December 2003). "Cholesterol depletion results in site-specific increases in epidermal growth factor receptor phosphorylation due to membrane level effects. Studies with cholesterol enantiomers". J. Biol. Chem. 278 (51): 51125–33. PMC 2593805
 . PMID 14530278. doi:10.1074/jbc.M304332200.
. PMID 14530278. doi:10.1074/jbc.M304332200. - ↑ Kristiana I, Luu W, Stevenson J, Cartland S, Jessup W, Belani JD, Rychnovsky SD, Brown AJ (September 2012). "Cholesterol through the looking glass: ability of its enantiomer also to elicit homeostatic responses". J. Biol. Chem. 287 (40): 33897–904. PMC 3460484
 . PMID 22869373. doi:10.1074/jbc.M112.360537.
. PMID 22869373. doi:10.1074/jbc.M112.360537.
External links
| Wikimedia Commons has media related to Cholesterol. |
- "About cholesterol". American Heart Association. Archived from the original on 3 October 2001.
- "Understanding the cholesterol test". Lab Tests Online. American Association for Clinical Chemistry.