Catumaxomab
| Monoclonal antibody | |
|---|---|
| Type | Trifunctional antibody |
| Source | Rat/mouse hybrid |
| Target | EpCAM, CD3 |
| Clinical data | |
| Trade names | Removab |
| AHFS/Drugs.com | International Drug Names |
| License data |
|
| Routes of administration | intraperitoneal infusion |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| ChemSpider |
|
| | |
Catumaxomab[1] (trade name Removab) is a rat-mouse hybrid monoclonal antibody which is used to treat malignant ascites, a condition occurring in patients with metastasizing cancer. It binds to antigens CD3 and EpCAM. It is in clinical trials in the United States currently and is used in Europe. It was developed by Fresenius Biotech and Trion Pharma (Germany).
History
Catumaxomab was developed by Trion Pharma, based on preliminary work by the Helmholtz Zentrum München. Dr. Horst Lindhofer is listed at the primary inventor of the patent.[2] Fresenius Biotech conducted clinical trials and filed the drug for approval with the European Medicines Agency (EMA). It was approved in Europe on 20 April 2009.[3]
Indications
The drug is approved for the treatment of malignant ascites in patients with EpCAM-positive cancer if a standard therapy is not available.[4][5] Ascites is an accumulation of fluid in the peritoneal cavity.
Application
The usual treatment of malignant ascites is to puncture the peritoneum to let the accumulated fluid drain out. After the puncture, catumaxomab is given as an intraperitoneal infusion. The procedure is repeated four times within about eleven days. It has been shown that puncture free survival can be increased from 11 to 46 days with this treatment.[6]
Adverse effects
Common adverse effects include fever, nausea and vomiting. Fever and pain should be controlled by giving NSAIDs, analgetics or antipyretics before application of catumaxomab.[7] All side effects were fully reversible in studies. Most are caused by the liberation of cytokines.[8]
Chemical structure
Catumaxomab consists of one "half" (one heavy chain and one light chain) of an anti-EpCAM antibody and one half of an anti-CD3 antibody, so that each molecule of catumaxomab can bind both EpCAM and CD3. In addition, the Fc-region can bind to an Fc receptor on accessory cells like other antibodies, which has led to calling the drug a trifunctional antibody.
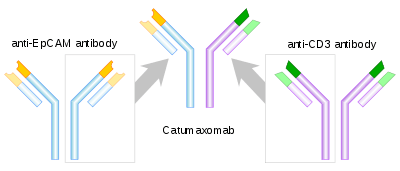
Mechanism of action
Many types of cancer cells carry EpCAM (epithelial cell adhesion molecule) on their surface. By binding to such a cell via one arm, to a T lymphocyte via the other arm and to an antigen-presenting cell like a macrophage, a natural killer cell or a dendritic cell via the heavy chains, an immunological reaction against the cancer cell is triggered. Removing cancer cells from the abdominal cavity reduces the tumour burden which is seen as the cause for ascites in cancer patients.[8][9][10]

References
- ↑ Linke, Rolf; Anke Klein; Diane Seimetz (2010). "Catumaxomab: Clinical development and future directions". MAbs. 2 (2): 129–136. PMC 2840231
 . PMID 20190561. doi:10.4161/mabs.2.2.11221.
. PMID 20190561. doi:10.4161/mabs.2.2.11221. - ↑ "About this file - European Patent Register". register.epo.org. Retrieved 2015-06-10.
- ↑ TRION Pharma: Trifunctional Antibody Catumaxomab Kills Cancer Stem Cells
- ↑ European Public Assessment Report for Removab
- ↑ Heiss u.a.: The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial. Int J Cancer. (2010) 127: 2209-2221
- ↑ Lordick, F; Ott, K; Weitz, J; Jäger, D (2008). "The evolving role of catumaxomab in gastric cancer". Expert Opinion on Biological Therapy. 8 (9): 1407–15. PMID 18694358. doi:10.1517/14712598.8.9.1407.
- ↑ Schubert-Zsilavecz, M, Wurglics, M, Neue Arzneimittel 2009
- 1 2 Assessment Report for Removab
- ↑ Capital Market Day Fresenius Biotech: Fresenius concentrates biotechnology activities on antibody and innovative cell therapies
- ↑ Ruf, P.; Gires, O.; J, M.; Fellinger, K.; Atz, J.; Lindhofer, H. (2007). "Characterisation of the new EpCAM-specific antibody HO-3: implications for trifunctional antibody immunotherapy of cancer". British Journal of Cancer. 97 (3): 315–321. PMC 2360319
 . PMID 17622246. doi:10.1038/sj.bjc.6603881.
. PMID 17622246. doi:10.1038/sj.bjc.6603881.
External links
- The trifunctional antibody catumaxomab: information for healthcare professionals (manufacturer website)