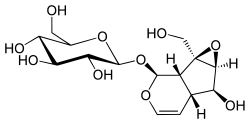
Catalpol
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| ECHA InfoCard | 100.017.568 |
| Chemical and physical data | |
| Formula | C15H22O10 |
| Molar mass | 362.33 g/mol |
| 3D model (JSmol) | |
| |
Catalpol is an iridoid glucoside. This natural product falls in the class of iridoid glycosides, which are simply monoterpenes with a glucose molecule attached. Although the pharmacological action of catalpol as well as many other iridoids has not been fully established, there is evidence that their primary function is to stimulate the production of adrenal cortical hormones, which increases the production of sex hormones. Catalpol also exhibits anti-inflammatory activity and has shown to increase the production of androgens yielded by the adrenal gland, which can lead to increases in muscle mass.[1]
Natural occurrences
First isolated in 1962, catalpol was named for plants in the genus Catalpa in which it was discovered. Later in 1969, catalpol was found to be present in larger quantities in several plants in genus Rehmannia (Orobanchaceae).[2]
It is found in plants belonging to several families, including, but not limited to, Scrophulariaceae, Lamiaceae (including scullcap[3][4]), Plantaginaceae (Plantago sp[5]) and Bignoniaceae,[6] all of which being in the order Lamiales.
Because they feed on these plants, Variable Checkerspot butterflies contain high amounts of catalpol,[7] which makes them unpalatable to predators and thus serves as a defense mechanism.[8]
Biosynthetic pathway
Though first isolated in the 1960s, there has been very little investigation of the biosynthetic pathway of catalpol.[6] S. R. Jensen has described a possible biosynthetic pathway for catalpol.[5] With iridoids stemming from a terpenoid origin, epi-iridotrial's precursor, epi-iridodial, is derived from geraniol.[9] Addition of a glucose at carbon 1 (C1) of the iridoid backbone and oxidation of the aldehyde at C4 of epi-iridotrial produced 8-epiloganic acid. A subsequent hydrolysis at C8 yielded mussaenosidic acid, followed by a dehydration to yield deoxyngeniposidic acid. The next precursor, geniposidic acid, was furnished via hydrolysis of C10, and then a decarboxylation to remove the carboxylic acid at C4 provided bartsioside. The very widely known and accepted precursor to catalpol, aucubin, was then furnished via hydroxylation at C6. Finally an epoxidation with the alcohol at C10 yielded the natural product, catalpol.[5]

Footnotes
- ↑ Chang, H-M (1986). Pharmacology and Applications of Chinese Materia Medica. Singapore: World Scientific.
- ↑ Tang, W. (1992). Chinese Drugs of Plant Origins. Berlin: Springer-Verlag. ISBN 0-387-19309-X.
- ↑ Phillipson, Carol A. Newall ; Linda A. Anderson ; J. David (1996). Herbal medicines : a guide for health care professionals (Reprinted. ed.). London: Pharmaceutical Press. p. 296. ISBN 0853692890.
- ↑ Yaghmai and Benson, 1979 M.S. Yaghmai, G.G. Benson The wax hydrocarbons of Scutellaria lateriflora L Manchester, England (1979) 228–229 p
- 1 2 3 Ronsted, N.; Gobel, E.; Franzyk, H.; Jansen, S. R.; Olsen, C. E. (2000). "Chemotaxonomy of Plantago . Iridoid glucosides and caffeoyl phenylethanoid glycosides". Phytochemistry. 55 (4): 337–48. PMID 11117882. doi:10.1016/S0031-9422(00)00306-X.
- 1 2 Damtoft, S. (1994). "Biosynthesis of Catalpol". Phytochemistry. 35 (5): 1187–9. doi:10.1016/S0031-9422(00)94819-2.
- ↑ Stermitz, Frank R., Maged S. Abdel-Kader, Tommaso A. Foderaro, and Marc Pomeroy. "Iridoid Glycosides from Some Butterflies and Their Larval Food Plants." Phytochemistry 37.4 (1994): 997-99.
- ↑ Bowers, M. D. "Unpalatability as a Defense Strategy of Western Checkerspot Butterflies (Euphydryas Scudder, Nymphalidae)." Evolution 35.2 (1981): 367-75.
- ↑ Jansen, S. R. (1991). "Plant iridoids, their biosynthesis and distribution in angiosperms". Ecological Chemistry and Biochemistry of Plant Terpenoids. Clarence Press. pp. 133–158.