Carbonic anhydrase II
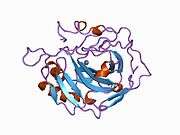
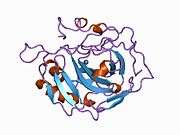
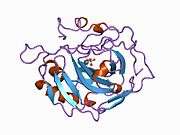
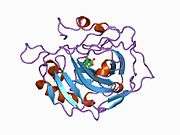
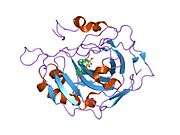
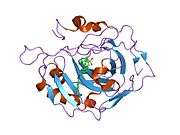
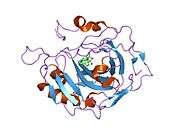
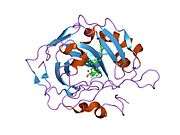
Carbonic anhydrase II (gene name CA2), is one of fourteen forms of human α carbonic anhydrases. Carbonic anhydrase catalyzes reversible hydration of carbon dioxide. Defects in this enzyme are associated with osteopetrosis and renal tubular acidosis. Renal carbonic anhydrase allows the reabsorption of bicarbonate ions in the proximal tubule. [3]
Interactions
Carbonic anhydrase II has been shown to interact with band 3[4][5][6][7] and sodium-hydrogen antiporter 1.[8]
References
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ "Entrez Gene: CA2 carbonic anhydrase II".
- ↑ Sterling, D; Reithmeier R A; Casey J R (December 2001). "A transport metabolon. Functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers". J. Biol. Chem. United States. 276 (51): 47886–94. ISSN 0021-9258. PMID 11606574. doi:10.1074/jbc.M105959200.
- ↑ Vince, J W; Reithmeier R A (October 1998). "Carbonic anhydrase II binds to the carboxyl terminus of human band 3, the erythrocyte C1-/HCO3- exchanger". J. Biol. Chem. UNITED STATES. 273 (43): 28430–7. ISSN 0021-9258. PMID 9774471. doi:10.1074/jbc.273.43.28430.
- ↑ Vince, J W; Carlsson U; Reithmeier R A (November 2000). "Localization of the Cl-/HCO3- anion exchanger binding site to the amino-terminal region of carbonic anhydrase II". Biochemistry. UNITED STATES. 39 (44): 13344–9. ISSN 0006-2960. PMID 11063570. doi:10.1021/bi0015111.
- ↑ Vince, J W; Reithmeier R A (May 2000). "Identification of the carbonic anhydrase II binding site in the Cl(-)/HCO(3)(-) anion exchanger AE1". Biochemistry. UNITED STATES. 39 (18): 5527–33. ISSN 0006-2960. PMID 10820026. doi:10.1021/bi992564p.
- ↑ Li, Xiuju; Alvarez Bernardo; Casey Joseph R; Reithmeier Reinhart A F; Fliegel Larry (September 2002). "Carbonic anhydrase II binds to and enhances activity of the Na+/H+ exchanger". J. Biol. Chem. United States. 277 (39): 36085–91. ISSN 0021-9258. PMID 12138085. doi:10.1074/jbc.M111952200.
Further reading
- Sly WS, Hu PY (1995). "Human carbonic anhydrases and carbonic anhydrase deficiencies.". Annu. Rev. Biochem. 64 (1): 375–401. PMID 7574487. doi:10.1146/annurev.bi.64.070195.002111.
- Kumpulainen T (1979). "Immunohistochemical localization of human carbonic anhydrase isoenzyme C.". Histochemistry. 62 (3): 271–80. PMID 114507. doi:10.1007/BF00508355.
- Henderson LE, Henriksson D, Nyman PO (1976). "Primary structure of human carbonic anhydrase C.". J. Biol. Chem. 251 (18): 5457–63. PMID 823150.
- Hu PY, Roth DE, Skaggs LA, et al. (1993). "A splice junction mutation in intron 2 of the carbonic anhydrase II gene of osteopetrosis patients from Arabic countries.". Hum. Mutat. 1 (4): 288–92. PMID 1301935. doi:10.1002/humu.1380010404.
- Roth DE, Venta PJ, Tashian RE, Sly WS (1992). "Molecular basis of human carbonic anhydrase II deficiency.". Proc. Natl. Acad. Sci. U.S.A. 89 (5): 1804–8. PMC 48541
 . PMID 1542674. doi:10.1073/pnas.89.5.1804.
. PMID 1542674. doi:10.1073/pnas.89.5.1804. - Dawson SJ, White LA (1992). "Treatment of Haemophilus aphrophilus endocarditis with ciprofloxacin.". J. Infect. 24 (3): 317–20. PMID 1602151. doi:10.1016/S0163-4453(05)80037-4.
- Schwartz GJ, Brion LP, Corey HE, Dorfman HD (1991). "Case report 668. Carbonic anhydrase II deficiency syndrome (osteopetrosis associated with renal tubular acidosis and cerebral calcification).". Skeletal Radiol. 20 (6): 447–52. PMID 1925679.
- Venta PJ, Welty RJ, Johnson TM, et al. (1991). "Carbonic anhydrase II deficiency syndrome in a Belgian family is caused by a point mutation at an invariant histidine residue (107 His----Tyr): complete structure of the normal human CA II gene.". Am. J. Hum. Genet. 49 (5): 1082–90. PMC 1683243
 . PMID 1928091.
. PMID 1928091. - Venta PJ, Tashian RE (1990). "PCR detection of the TAQ1 polymorphism at the CA2 locus.". Nucleic Acids Res. 18 (18): 5585. PMC 332284
 . PMID 1977133. doi:10.1093/nar/18.18.5585.
. PMID 1977133. doi:10.1093/nar/18.18.5585. - Sato S, Zhu XL, Sly WS (1990). "Carbonic anhydrase isozymes IV and II in urinary membranes from carbonic anhydrase II-deficient patients.". Proc. Natl. Acad. Sci. U.S.A. 87 (16): 6073–6. PMC 54474
 . PMID 2117271. doi:10.1073/pnas.87.16.6073.
. PMID 2117271. doi:10.1073/pnas.87.16.6073. - Kaunisto K, Parkkila S, Tammela T, et al. (1990). "Immunohistochemical localization of carbonic anhydrase isoenzymes in the human male reproductive tract.". Histochemistry. 94 (4): 381–6. PMID 2121671. doi:10.1007/BF00266444.
- Backman U, Danielsson B, Wistrand PJ (1991). "The excretion of carbonic anhydrase isozymes CA I and CA II in the urine of apparently healthy subjects and in patients with kidney disease.". Scand. J. Clin. Lab. Invest. 50 (6): 627–33. PMID 2123360. doi:10.3109/00365519009089180.
- Forsman C, Behravan G, Osterman A, Jonsson BH (1989). "Production of active human carbonic anhydrase II in E. coli.". Acta Chemica Scandinavica B. 42 (5): 314–8. PMID 2850697.
- Venta PJ, Montgomery JC, Hewett-Emmett D, Tashian RE (1986). "Comparison of the 5' regions of human and mouse carbonic anhydrase II genes and identification of possible regulatory elements.". Biochim. Biophys. Acta. 826 (4): 195–201. PMID 3000449. doi:10.1016/0167-4781(85)90006-5.
- Ohlsson A, Cumming WA, Paul A, Sly WS (1986). "Carbonic anhydrase II deficiency syndrome: recessive osteopetrosis with renal tubular acidosis and cerebral calcification.". Pediatrics. 77 (3): 371–81. PMID 3081869.
- Nakai H, Byers MG, Venta PJ, et al. (1987). "The gene for human carbonic anhydrase II (CA2) is located at chromosome 8q22.". Cytogenet. Cell Genet. 44 (4): 234–5. PMID 3107918. doi:10.1159/000132378.
- Montgomery JC, Venta PJ, Tashian RE, Hewett-Emmett D (1987). "Nucleotide sequence of human liver carbonic anhydrase II cDNA.". Nucleic Acids Res. 15 (11): 4687. PMC 340889
 . PMID 3108857. doi:10.1093/nar/15.11.4687.
. PMID 3108857. doi:10.1093/nar/15.11.4687. - Murakami H, Marelich GP, Grubb JH, et al. (1988). "Cloning, expression, and sequence homologies of cDNA for human carbonic anhydrase II.". Genomics. 1 (2): 159–66. PMID 3121496. doi:10.1016/0888-7543(87)90008-5.
- Eriksson AE, Jones TA, Liljas A (1989). "Refined structure of human carbonic anhydrase II at 2.0 A resolution.". Proteins. 4 (4): 274–82. PMID 3151019. doi:10.1002/prot.340040406.
- Eriksson AE, Kylsten PM, Jones TA, Liljas A (1989). "Crystallographic studies of inhibitor binding sites in human carbonic anhydrase II: a pentacoordinated binding of the SCN- ion to the zinc at high pH.". Proteins. 4 (4): 283–93. PMID 3151020. doi:10.1002/prot.340040407.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.