Carbene radical
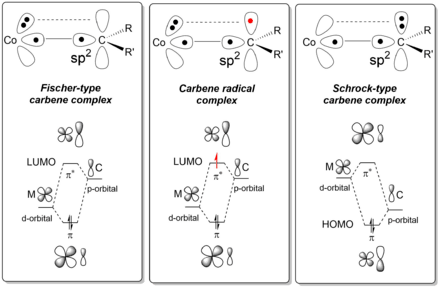
Carbene radicals are a special class of organometallic carbenes. The carbene radical can be formed by one-electron reduction of Fischer-type carbenes using an external reducing agent, or directly upon carbene formation at an open-shell transition metal complex (in particular low-spin cobalt(II) complexes) using diazo compounds and related carbene precursors.[1] Cobalt(III)-carbene radicals have found catalytic applications in cyclopropanation reactions,[2][3][4] as well as in a variety of other catalytic radical-type ring-closing reactions. [5] [6] [7][8]
Theoretical calculations and EPR studies confirmed their radical-type behaviour and explained the bonding interactions underlying the stability of the carbene radical.[9] [10] Stable carbene radicals of other metals are known,[1] but the catalytically relevant cobalt(III)-carbene radicals have thus far only been synthesized as long-lived reactive intermediates.[11][12]

Bonding interactions and Radical Reactivity
The chemical bond present in carbene radicals is surprising in that it possesses aspects of both Fischer and Schrock type carbenes.[1][9][10] As a result, the cobalt carbene radical complexes have discrete radical-character at their carbon atom, thus giving rise to interesting catalytic radical-type reaction pathways.
The mechanism of formation of a carbene radical at cobalt(II) typically involves carbene generation at the metal with simultaneous intramolecular electron transfer from the metal into the metal-carbene π* anti-bonding molecular orbital constructed from the metal d-orbital and the carbene p-orbital. As such, carbene radicals are perhaps best described as 'one-electron reduced Fischer-type carbenes'.[1]
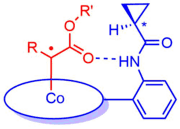
Discrete electron transfer from a sigma-type metal d-orbital (typically the dz2 orbital) occurs,[1][10] leads the typical radical character of the carbene carbon. This behaviour not only explains the carbon-centered radical-type reactivity of these complexes, but also their reduced electrophilicity (suppressing carbene-carbene dimerisation side reactions) as well as their enhanced reactivity to electron-deficient substrates. Furthermore, second coordination sphere hydrogen-bonding interactions give rise to faster reactions because H-bonds are stronger to the reduced carbene as compared to the precursor.[9] Such H-bonding interactions can also facilitate chirality transfer in enantioselective carbene-transfer reactions.[13]
See also
References
- 1 2 3 4 5 Dzik, W.I.; Zhang, X.P.; de Bruin, B. (2011). "The Redox Non-Innocence of Carbene Ligands: Carbene Radicals in (Catalytic) C-C Bond Formation". Inorganic Chemistry. 50 (20): 9896–9903. doi:10.1021/ic200043a.
- ↑ Ikeno, T.; Iwakura, I.; Yamada, T. (2002). "Cobalt−Carbene Complex with Single-Bond Character: Intermediate for the Cobalt Complex-Catalyzed Cyclopropanation". Journal of the American Chemical Society. 124 (51): 15152–15153. doi:10.1021/ja027713x.
- ↑ Huang, L.; Chen, Y.; Gao, G.-Y.; Zhang, X.P. (2003). "Diastereoselective and enantioselective cyclopropanation of alkenes catalyzed by cobalt porphyrins". Journal of Organic Chemistry. 68 (21): 8179–8184. doi:10.1021/jo035088o.
- ↑ Chirila, A.; Das, B.G.; Paul, N.D.; de Bruin, B. (2017). "Diastereoselective Radical-Type Cyclopropanation of Electron Deficient Alkenes mediated by the Highly Active [Co(MeTAA)] Catalyst". ChemCatChem. doi:10.1002/cctc.201601568.
- ↑ Das, B.G.; Chirila, A.; Tromp, M.; Reek, J.N.H.; de Bruin, B. (2016). "Co(III)-Carbene Radical Approach to Substituted 1H-Indenes". Journal of the American Chemical Society. 138 (28): 8968–8975. doi:10.1021/jacs.6b05434.
- ↑ Cui, X.; Xu, X.; Jin, L.-M.; Wojtas, L.; Zhang, X.P. (2017). "Stereoselective Radical C–H Alkylation with Acceptor/Acceptor-Substituted Diazo Reagents via Co(II)-Based Metalloradical Catalysis". Chemical Science. 6: 1219–1224. doi:10.1039/C4SC02610A.
- ↑ Paul, N.D.; Chirila, A.; Lu, H.; Zhang, X.P.; de Bruin, B. (2013). "Carbene Radicals in Cobalt(II) Porphyrin-Catalysed Carbene Carbonylation Reactions; A Catalytic Approach to Ketenes". Chemistry European Journal. 19 (39): 12953–12958. doi:10.1002/chem.201301731.
- ↑ Paul, N.D.; Mandal, S.; Otte, M.; Cui, M.; Zhang, X.P.; de Bruin, B. (2014). "A Metalloradical Approach to 2H-Chromenes". Journal of the American Chemical Society. 136 (3): 1090–1096. doi:10.1021/ja4111336.
- 1 2 3 Dzik, W.I.; Xu, X.; Zhang, X.P.; Reek, J.N.H.; de Bruin, B. (2010). "'Carbene Radicals' in CoII(por)-Catalyzed Olefin Cyclopropanation". Journal of the American Chemical Society. 132 (31): 10891–10902. doi:10.1021/ja103768r.
- 1 2 3 Belof, J.; Cioce, C.; Xu, X.; Zhang, X.P.; Space, B.; Woodcock, H.L. (2011). "Characterization of tunable radical metal–carbenes: Key intermediates in catalytic cyclopropanation". Organometallics. 30 (10): 2739–2746. doi:10.1021/om2001348.
- ↑ Lu, H.; Dzik, W.; Xu, X.; Wojtas, L.; de Bruin, B.; Zhang, X.P. (2011). "Experimental evidence for cobalt(III)-carbene radicals: Key intermediates in cobalt(II)-based metalloradical cyclopropanation". Journal of the American Chemical Society. 133 (22): 8518–8521. PMID 21563829. doi:10.1021/ja203434c.
- ↑ Russell, S.; Hoyt, J.; Bart, S.; Milsmann, C.; Stieber, S.C.; Semproni, S.; DeBeer, S.; Chirik, P. (2014). "Synthesis, electronic structure and reactivity of bis(imino)pyridine iron carbene complexes: evidence for a carbene radical". Chemical Science. 5: 1168–1174. doi:10.1039/C3SC52450G.
- ↑ Xu, X.; Zhu, S.; Cui, X.; Wojtas, L.; Zhang, X.P. (2013). "Cobalt(II)-Catalyzed Asymmetric Olefin Cyclopropanation with α-Ketodiazoacetates". Angewandte Chemie International Edition. 52 (45): 11857–11861. doi:10.1002/anie.201305883.