Capillary electrophoresis–mass spectrometry

Capillary electrophoresis–mass spectrometry (CE-MS) is an analytical chemistry technique formed by the combination of the liquid separation process of capillary electrophoresis with mass spectrometry.[1] CE-MS combines advantages of both CE and MS to provide high separation efficiency and molecular mass information in a single analysis.[2] It has high resolving power and sensitivity, requires minimal volume and can analyze at high speed. Ions are typically formed by electrospray ionization,[3] but they can also be formed by matrix-assisted laser desorption/ionization[4] or other ionization techniques. It has applications in basic research in proteomics[5] and quantitative analysis of biomolecules[6] as well as in clinical medicine.[7][8]
Since its introduction in 1987, new developments and application has made CE-MS powerful separation and identification technique. Use of CE-MS has increased for protein and peptides analysis and other biomolecules. Understanding of CE, the interface setup, ionization technique and mass detection system is important to tackle problems while coupling capillary electrophoresis to mass spectrometry.
History
The original interface between capillary zone electrophoresis and mass spectrometry was developed in 1987[9] by Richard D. Smith and coworkers at Pacific Northwest National Laboratory, and who also later were involved in development of interfaces with other CE variants, including capillary isotachophoresis and capillary isoelectric focusing.
Interfacing CE with MS
Capillary electrophoresis is a separation technique which uses high electric field to produce electroosmotic flow for separation of ions. Analytes migrate from one end of capillary to other based on their charge, viscosity and size. Higher the electric field, greater is the mobility. Mass spectrometry is an analytical technique that identifies chemical species depending on their mass-to-charge ratio. During the process, an ion source will convert molecules coming from CE to ions that can then be manipulated using electric and magnetic field. The separated ions are then measured using a detector. The major problem faced when coupling CE to MS arises due to insufficient understanding of fundamental processes when two techniques are interfaced. The separation and detection of analytes can be improved with better interface. CE has been coupled to MS using various ionization techniques like FAB, ESI, MALDI, APCI and DESI. The most used ionization technique is ESI.
Electrospray ionization interface
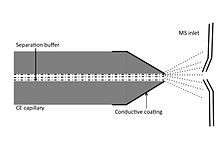
The first CE-MS interface had cathode end of CE capillary terminated within a stainless steel capillary. An electrical contact was made at that point completing the circuit and initiating the electrospray. This interface system had few drawbacks like mismatch in the flow rates of two systems. Since then, interface system has been improved to have continuous flow rate and good electrical contact. At present, three types of interface system exist for CE/ESI-MS which are discussed briefly.
Sheathless interface
CE capillary is coupled directly to ionization source in sheathless interface system. The capillary is coated with conducting metal or gold or platinum wire is inserted into CE capillary to establish suitable electrical connection. Since no sheath liquid is used, the system has high sensitivity, low flow rates and minimum background. However, right choice of buffer solution has to be made which is suitable for both CE separation and ESI operation.
Sheath flow interface

It is most widely used interface system. The electrical connection is established when the CE separation liquid is mixed with sheath liquid flowing coaxially in a metal capillary tubing. Commonly used sheath liquid is 1:1 mixture of water-methanol with 0.1% acetic acid or formic acid. The system is more reliable and has wide selection range of separation electrolyte. There might be some decrease in sensitivity due to sheath liquid.
Liquid junction interface
This technique uses a stainless steel tee to mix separation electrolyte from CE capillary with make up liquid. The CE capillary and ESI needle are inserted through opposite sides of the tee and a narrow gap is maintained. The electrical contact is established by makeup liquid surrounding the junction between two capillaries. This system is easy to operate. However, the sensitivity is reduced and the mixing of two liquids could degrade separation.
Continuous- flow fast atom bombardment
Miniard et al. first coupled FAB to CE in 1988. The main problem of this interface is the flow rate between the two systems. The CF-FAB needs very high flow rate but CE need very minimal flow rate for better separation. So a make up flow is introduced using the designs discussed above: sheath flow or liquid junction. CF/FAB-MS is not commonly used as CE/ESI-MS, as FAB is rarely used anymore.
Coupling CE with MALDI-MS

Off-line coupling of CE to MALDI, the CE effluent could be sprayed or added drop wise on MALDI target plate then dried and analyzed by MS. For online coupling, a moving target with continuous contact to CE capillary end is required. The moving target takes analytes into MS where it is desorbed and ionized. Musyimi et al. developed a new technique where rotating ball was used to transfer CE to MS. The sample from CE is mixed with matrix coming though another capillary. As the ball rotates the sample is dried before it reaches ionization region. This technique has high sensitivity since no makeup fluid is used.[10]
Applications
CE-MS ability to separate analytes present in extremely low concentration with high efficiency at high speed has made it applicable in all fields of science. CE-MS has been used for bioanalytical, pharmaceuticals, environmental and forensic application. The major application of CE-MS has been for biological studies, mostly for protein and peptide analysis. Along with that, it is used often for routine analysis of pharmaceutical drugs. There are number of studies reporting characterization of mixtures of peptides and proteins.CE-MS can be used for routine clinical checkup. Body fluids like blood and urine have been analyzed with CE-MS to identify biomarkers for renal diseases and cancer.[11]
See also
References
- ↑ Loo JA, Udseth HR, Smith RD (June 1989). "Peptide and protein analysis by electrospray ionization-mass spectrometry and capillary electrophoresis-mass spectrometry". Anal. Biochem. 179 (2): 404–12. PMID 2774189. doi:10.1016/0003-2697(89)90153-X.
- ↑ Cai, Jianyi; Henion, Jack (1995). "Capillary electrophoresis-mass spectrometry". Journal of Chromatography A. 703: 667–692. doi:10.1016/0021-9673(94)01178-h.
- ↑ Maxwell EJ, Chen DD (October 2008). "Twenty years of interface development for capillary electrophoresis-electrospray ionization-mass spectrometry". Anal. Chim. Acta. 627 (1): 25–33. PMID 18790125. doi:10.1016/j.aca.2008.06.034.
- ↑ Zhang H, Caprioli RM (September 1996). "Capillary electrophoresis combined with matrix-assisted laser desorption/ionization mass spectrometry; continuous sample deposition on a matrix-precoated membrane target". J Mass Spectrom. 31 (9): 1039–46. PMID 8831154. doi:10.1002/(SICI)1096-9888(199609)31:9<1039::AID-JMS398>3.0.CO;2-F.
- ↑ Metzger J, Schanstra JP, Mischak H (August 2008). "Capillary electrophoresis-mass spectrometry in urinary proteome analysis: current applications and future developments". Anal Bioanal Chem. 393 (5): 1431–42. PMID 18704377. doi:10.1007/s00216-008-2309-0.
- ↑ Ohnesorge J, Neusüss C, Wätzig H (November 2005). "Quantitation in capillary electrophoresis-mass spectrometry". Electrophoresis. 26 (21): 3973–87. PMID 16252322. doi:10.1002/elps.200500398.
- ↑ Kolch W, Neusüss C, Pelzing M, Mischak H (2005). "Capillary electrophoresis-mass spectrometry as a powerful tool in clinical diagnosis and biomarker discovery". Mass Spectrom Rev. 24 (6): 959–77. PMID 15747373. doi:10.1002/mas.20051.
- ↑ Dakna M, He Z, Yu WC, Mischak H, Kolch W (November 2008). "Technical, bioinformatical and statistical aspects of liquid chromatography-mass spectrometry (LC-MS) and capillary electrophoresis-mass spectrometry (CE-MS) based clinical proteomics: A critical assessment". J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877 (13): 1250–8. PMID 19010091. doi:10.1016/j.jchromb.2008.10.048.
- ↑ Schmitt-Kopplin, P., Frommberger, M.(2003).Capillary electrophoresis – mass spectrometry: 15 years of developments and applications. Electrophoresis, 24, 3837-3867.
- ↑ Musyimi H.K.; Narcisse D. A.; Zhang X.; Stryjewski, W.; Soper S. A.; Murray K. K. (2004) “Online CE-MALDI –TOF MS using a rotating ball interface.” Anal Chem 76:5968-5973
- ↑ Mischak H.; Coon J.J.; Novak J.; Weissinger E. M.; Schanstra J.P.; Dominiczak A.F.Capillary electrophoresis-mass spectrometry as a powerful tool in biomarker discovery and clinical diagnosis: an update of recent developments. Mass Spec. Reviews. 28(2008)