Chacma baboon
| Chacma baboon[1] | |
|---|---|
 | |
| A male gray-footed chacma, one of the subspecies of chacma baboon | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Primates |
| Family: | Cercopithecidae |
| Genus: | Papio |
| Species: | P. ursinus |
| Binomial name | |
| Papio ursinus (Kerr, 1792) | |
| Subspecies | |
|
3 ssp., see text | |
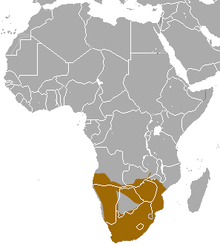 | |
| Geographic range | |
The Chacma baboon (Papio ursinus), also known as the Cape baboon, is, like all other baboons, from the Old World monkey family. It is one of the largest of all monkeys.
Located primarily in southern Africa, the chacma baboon has a wide variety of social behaviors, including a dominance hierarchy, collective foraging, adoption of young by females, and friendship pairings. These behaviors form parts of a complex evolutionary ecology.
In general the species is not threatened, but human population pressure has increased contact between humans and baboons. Hunting, accidents and trapping kill or remove many baboons from the wild. This has reduced baboon numbers and disrupted their social structure.
Taxonomy
Due to hybridization between different baboon (Papio) populations across Africa, authors have occasionally grouped the entire radiation as a single species, the hamadryas baboon, Papio hamadryas. Arbitrary boundaries were then used to separate the populations into subspecies.[3] Other authors considered the chacma baboon a subspecies of the yellow baboon, Papio cynocephalus, though it is now recognised as a separate species, Papio ursinus. The chacma baboon has two or three subspecies, depending on which classification is followed. Grubb et al. (2003) listed two subspecies,[4] while Groves (2005) in Mammal Species of the World listed three.[1] This article follows Groves (2005) and describes three distinct subspecies. In the Grubb et al. (2003) paper, P. u. raucana was believed to be synonymous with P. u. ursinus.[4]
- Papio ursinus ursinus Kerr, 1792 – Cape chacma (found in southern South Africa)
- P. u. griseipes Pocock, 1911 – Gray-footed chacma (found in northern South Africa to southern Zambia)
- P. u. raucana Shortridge, 1942 – Ruacana chacma (found from Namibia to southern Angola)
Physical description
The chacma baboon is perhaps the longest species of monkey, with a male body length of 50–115 cm (20–45 in) and tail length of 45–84 cm (18–33 in).[5][6] It also one of the heaviest; the male weighs from 21 to 45 kg (46 to 99 lb) with an average of 31.8 kg (70 lb). Baboons are sexually dimorphic, and females are considerably smaller than males. The female chacma weighs from 12 to 25 kg (26 to 55 lb), with an average of 15.4 kg (34 lb).[7][8][9][10] It is similar in size to the olive baboon, averaging slightly higher in mean body mass, and of similar weight to the more compact mandrill, the males of which weigh on average about 1 kg (2.2 lb) more than a chacma baboon, the females weigh 3 kg (6.6 lb) less than the female chacma. While the mandrill is usually crowned the largest of all modern monkeys, going on total length and average (but not maximum) body weight between the sexes, the chacma baboon appears to be the largest extant monkey.[9][11] The chacma baboon is generally dark brown to gray in color, with a patch of rough hair on the nape of its neck. Unlike the males of northern baboon species (the Guinea, hamadryas, and olive baboons), chacma males do not have a mane. Perhaps the most distinctive feature of this baboon is its long, downward-sloping face.[12][13] The canine teeth of male chacma baboons have a mean length of 3.86 ± 0.30 cm at the time they emigrate from their natal troop. This is the time of greatest tooth length as the teeth tend to wear or be broken thereafter.[14]
The three subspecies are differentiated by size and color. The Cape chacma is a large, heavy, dark-brown, and has black feet. The gray-footed chacma is slightly smaller than the Cape chacma, lighter in color and build, and has gray feet. The Ruacana chacma generally appears to be a smaller, less darkly colored version of the Cape chacma.
Ecology
Habitat and distribution
The chacma baboon inhabits a wide array of habitats including woodland, savanna, steppes, and subdesert, from the grassy alpine slopes of the Drakensberg to the Kalahari desert.[2] During the night the chacma baboon needs hills, cliffs, or large trees in which to sleep. During the day water availability may limit its range in arid areas.[2] It is found in southern Africa, ranging from South Africa north to Angola, Zambia, and Mozambique. The subspecies are divided across this range. The Cape chacma is found in southern South Africa; the gray-footed chacma, is present from northern South Africa, through the Okavango Delta in Botswana, Zimbabwe, Mozambique (south of the Zambezi), to southwest Zambia; and the Ruacana chacma is found in northern Namibia and southern Angola.[2]
 Subspecies (P. u. ursinus), is found in southern South Africa
Subspecies (P. u. ursinus), is found in southern South Africa
Diet
The chacma baboon is omnivorous with a preference for fruits, while also eating insects, seeds, grass, smaller vertebrate animals, and fungi (the desert truffle Kalaharituber pfeilii;[15] at the Cape of Good Hope in particular, it is also known for taking shellfish and other marine invertebrates.[16] It is generally a scavenger when it comes to game meat, and rarely engages in hunting large animals. One incident of a chacma baboon killing a human infant has been reported, but the event is so rare, the locals believed it was due to witchcraft.[17] Normally, chacma baboons will flee at the approach of humans, though this is changing due to the easy availability of food near human dwellings.[18]
Behavior
Social organization
 |
Baboon call
A dominant male baboon calls to his pride |
| Problems playing this file? See media help. | |
The chacma baboon usually lives in social groups, called troops, which are composed of multiple adult males, adult females, and their offspring. Occasionally, however, very small groups form that consist of only a single adult male and several adult females.[19] Chacma troops are characterized by a dominance hierarchy. Female ranking within the troop is inherited through the mother and remains relatively fixed, while male ranking is often in flux, especially when the dominant male is replaced. Chacmas are unusual among baboons in that neither males nor females form strong relationships with members of the same sex. Instead, the strongest social bonds are often between unrelated adult males and females. Infanticide is also common compared to other baboon species, as newly dominant males will often attempt to kill young baboons sired by the previously dominant male.[20] Baboon troops possess a complex group behavior and communicate by means of body attitudes, facial expressions, vocalizations and touch.
Morning dispersal patterns
The chacma baboon often sleeps in large groups on cliffs or in trees at night to avoid predators. The morning dispersal from the sleeping site is synchronized, with all members leaving at the same time. In most cases, dispersal is initiated by a single individual, and the other members of the group decide whether or not to follow. At least five followers must be recruited for a successful dispersal initiation, and not all initiation attempts are successful. Surprisingly, the initiator's dominance status shows little correlation with successful initiation of departure; more-dominant individuals are no more likely to lead a successful departure than subordinate individuals. One study has shown that while the success rate of dispersal initiation attempts is relatively constant across all sexes, male are more likely to attempt initiation than females, and lactating females are less likely to attempt initiation than females without dependent offspring.[21] A separate study has achieved slightly different results. While dominance hierarchy does not play a significant role in initiating the morning dispersal, social affiliation does. Chacma baboons that play a more central role in the group (as measured by grooming behavior and time spent with other members) are more likely to be followed during the morning dispersal. This study concluded that group members are more likely to follow the behavior of individuals with which they are closely affiliated.[22]
Foraging behavior

Dominance does play a role in group foraging decisions. A dominant individual (usually the alpha male) leads the group to easily monopolized resources. The group usually follows, even though many subordinate members cannot gain access to that particular resource. As in morning dispersal, the inclination of group members to follow the leader is positively associated with social interactions with that dominant individual.[23]
Collective foraging behavior, with many individuals taking advantage of the same resource at once, has also been observed. However, this behavior can be chiefly attributed to shared dietary needs rather than social affiliation. Pregnant females, who share similar dietary needs, are more likely to synchronize their behavior than fertile females. Foraging synchronization decreases in areas with lower food density.[24]
Adoption
Adoption behavior has been observed in chacma baboons. Orphaned baboons whose mothers have disappeared or died are often too small to care for themselves. In one study of nine natural orphans and three introduced orphans, all but one orphan were adopted by another member of the group. The individual that was not adopted was 16 months old, four months older than the next oldest orphan, and was old enough to survive on its own. Adoption behavior includes sleeping close to the orphaned infant, grooming and carrying the orphan, and protecting it from harassment by other members of the troop. Both males and females care for infants, and care does not depend on the infant's sex. Additionally, all caregivers are prereproductive, only four or five years of age. The two major theories explaining this behavior are kin selection, in which caregivers take care of potentially related orphans, and parental practice, in which young caregivers increase their own fitness by using an orphan to practice their own parental skills.[25]
Friendship
Males and female chacma baboons often form relationships referred to as "friendships". These cooperative relationships generally occur between lactating females and adult males. The females are believed to seek out male friendships to gain protection from infanticide. In many baboon species, immigrant alpha males often practice infanticide upon arrival in a new troop. By killing unrelated infants, the new male shortens the time until he can mate with the females of the troop. A female with dependent offspring generally does not become sexually receptive until she weans her offspring at around 12 months of age. However, a mother usually becomes sexually receptive shortly after the death of her offspring.[26]

This protection hypothesis is supported by studies of stress hormones in female baboons during changes in the male hierarchy. When an immigrant male ascends to the top of the male dominance hierarchy, stress hormones in lactating and pregnant females increases, while stress hormones in females not at risk of infanticide stay the same. Additionally, females in friendships with males exhibit a smaller rise in stress hormones than do females without male friends.[27]
The benefits of friendship to males are less clear. A male is more likely to enter into friendships with females with which he has mated, which indicates males might enter into friendships to protect their own offspring and not just to protect that female's future reproductive success.[28] These friendships may play a role in the mating system of chacma baboons. A female will often mate with several males, which increases the number of potential fathers for her offspring and increases the chances she will be able to find at least one friend to protect her infants.[29]
Female chacma baboons have been observed to compete with each other for male friends. This may be the result of one male having a high probability of paternity with multiple females. These competitions are heavily influenced by the female dominance hierarchy, with dominant females displacing subordinate females in friendships with males. Generally, when a more-dominant female attempts to make friends with an individual which is already the friend of a subordinate female, the subordinate female reduces grooming and spatial proximity to that male, potentially leaving her offspring at higher risk of infanticide.[30]
Relationship with humans
Conservation
The chacma baboon is widespread and does not rank among threatened animal species. However, in some confined locations, such as South Africa's Southern Cape Peninsula, local populations are dwindling due to habitat loss and predation from other protected species, such as leopards and lions.[31] Some troops have become a suburban menace, overturning trash cans and entering houses in their search for food. These troops can be aggressive and dangerous, and such negative encounters have resulted in hunting by frustrated local residents. This isolated population is thought to face extinction within 10 years.[18]

The chacma is listed under Appendix II of CITES as it occurs in many protected areas across its range.[2] The only area in South Africa where they are monitored is in the Cape Peninsula, where they are protected.
Observations by those working hands-on in South Africa's rehabilitation centers have found this species is damaged by human intervention; troop structures are influenced, and over the years a significant loss in numbers has occurred. Because they live near human habitats, baboons are shot, poisoned, electrocuted, run over, and captured for the pet industry, research laboratories and muthi (medicine).[32] Despite this, assessors working for the IUCN believe there are no major threats that could result in a range-wide decline of the species.[2]
See also
References
- 1 2 Groves, C.P. (2005). Wilson, D.E.; Reeder, D.M., eds. Mammal Species of the World: A Taxonomic and Geographic Reference (3rd ed.). Baltimore: Johns Hopkins University Press. p. 167. ISBN 0-801-88221-4. OCLC 62265494.
- 1 2 3 4 5 6 Hoffmann, M. & Hilton-Taylor, C. (2008). "Papio ursinus". IUCN Red List of Threatened Species. Version 2008. International Union for Conservation of Nature. Retrieved 2009-01-04.
- ↑ Fleagle, J. (1999). Primate Adaptation and Evolution (2nd ed.). Academic Press. p. 195. ISBN 0-12-260341-9.
- 1 2 Grubb, P.; Butynski, T. M.; Oates, J. F.; Bearder, S. K.; Disotell, T. R.; Groves, C. P.; Struhsaker, T. T. (2003). "Assessment of the diversity of African primates". International Journal of Primatology. 24 (6): 1301–1357. doi:10.1023/B:IJOP.0000005994.86792.b9.
- ↑ Nowak, R.M. (1991). Walker’s Mammals of the World. Baltimore and London: The Johns Hopkins University Press. ISBN 9780801857898.
- ↑ Estes, R.D. (1992). The Behavior Guide To African Mammals: Including Hoofed Mammals, Carnivores, Primates. Berkeley, California: University of California Press. ISBN 9780520080850.
- ↑ Burnie, D.; Wilson, D. E. (2005). Animal: The Definitive Visual Guide to the World's Wildlife. DK Adult. ISBN 0-7894-7764-5.
- ↑ Kingdon, J. (1993). Kingdon Guide to African Mammals. ISBN 978-0-85112-235-9.
- 1 2 Kingdon, J., Happold, D., Butynski, T., Hoffmann, M., Happold, M., & Kalina, J. (2013). Mammals of Africa (Vol. 1). A&C Black.
- ↑ Coid, C., Poilecot, P., & de Zborowski, I. (2002). Les hommes et les animaux dans la moyenne vallée du Zambèze, Zimbabwe. Editions Quae.
- ↑ Dechow, P. C. (1983). "Estimation of body weights from craniometric variables in baboons" (PDF). American Journal of Physical Anthropology. 60 (1): 113–123. PMID 6869499. doi:10.1002/ajpa.1330600116.
- ↑ Jolly, C. J. (1993). W. H. Kimbel; L. B. Martins, eds. Species, Species Concepts, and Primate Evolution. New York: Plenum Press. ISBN 9780306442971.
- ↑ Groves, C. (2001). Primate Taxonomy. Washington DC: Smithsonian Institution Press. ISBN 9781560988724.
- ↑ Hamilton, W. J.; Bulger, J. B. (1990). "Natal male baboon rank rises and successful challenges to resident alpha males". Behavioral Ecology and Sociobiology. 26 (5): 357–362. JSTOR 4600418. doi:10.1007/BF00171102.
- ↑ Trappe JM, Claridge AW, Arora D, Smit WA (2008). "Desert truffles of the Kalahari: ecology, ethnomycology and taxonomy". Economic Botany. 62 (3): 521–529. doi:10.1007/s12231-008-9027-6.
- ↑ Davidge, C. (1978). "Ecology of baboons (Papio ursinus) at Cape Point". Zoologica Africana. 13 (2): 329–350. doi:10.1080/00445096.1978.11447633.
- ↑ Dhlamini, D. (6 August 2003). "Witchcraft, says baby's mom". News24. Retrieved 15 December 2007.
- 1 2 Thomas, L. (2006). "Working to Save South Africa's Chacma Baboons". GoNOMAD. Retrieved 19 February 2007.
- ↑ Byrne, R. W.; Whiten, A.; Henzi, S. P. (1987). "One male groups and intertroop interactions of mountain baboons". International Journal of Primatology. 8: 615–633. doi:10.1007/BF02735780.
- ↑ Henzi, P.; Barrett, L. (2003). "Evolutionary ecology, sexual conflict, and behavioral differentiation among baboon populations". Evolutionary Anthropology. 12: 217–230. doi:10.1002/evan.10121.
- ↑ Stueckle, S.; Zinner, D. (2008). "To follow or not to follow: decision making and leadership during the morning departure in chacma baboons". Animal Behaviour. 75: 1995–2004. doi:10.1016/j.anbehav.2007.12.012.
- ↑ King, A. J.; Sueur, C.; Huchard, E.; Cowlishaw, G. (2011). "A rule-of-thumb based on social affiliation explains collective movements in desert baboons". Animal Behaviour. 82: 1337–1345. doi:10.1016/j.anbehav.2011.09.017.
- ↑ King, A. J.; Douglas, C. M. S.; Huchard, E.; Isaac, N. J. B.; Cowlishaw, G. (2008). "Dominance and affiliation mediate despotism in a social primate". Current Biology. 18 (23): 1833–1838. PMID 19026539. doi:10.1016/j.cub.2008.10.048.
- ↑ King, A. J.; Cowlishaw, G. (2009). "All together now: behavioural synchrony in baboons". Animal Behaviour. 78: 1381–1387. doi:10.1016/j.anbehav.2009.09.009.
- ↑ Hamilton, W. J.; Busse, C.; Smith, K. S. (1982). "Adoption of infant orphan chacma baboons". Animal Behaviour. 30: 29–34. doi:10.1016/S0003-3472(82)80233-9.
- ↑ Engh, A. L.; Beehner, J. J.; Bergman, T. J.; Whitten, P. L.; Hoffmeiers, R. K.; Seyfarth, R. M.; Cheney, D. L. (2006). "Female hierarchy instability, male immigration and infanticide increase glucocorticoid levels in female chacma baboons" (PDF). Animal Behaviour. 71: 1227–1237. doi:10.1016/j.anbehav.2005.11.009.
- ↑ Beehner, J. C.; Bergman, T. J.; Cheney, D. L.; Seyfarth, R. M; Whittens, P. L. (2005). "The effect of new alpha males on female stress in free-ranging baboons" (PDF). Animal Behaviour. 69: 1211–1221. doi:10.1016/j.anbehav.2004.08.014.
- ↑ Moscovice, L. R.; Di Fiore, A.; Crockford, C.; Kitchen, D. M.; Wittig, R.; Seyfarth, R. M.; Cheney, D. L. (2010). "Hedging their bets? Male and female chacma baboons form friendships based on likelihood of paternity" (PDF). Animal Behaviour. 79: 1007–1015. doi:10.1016/j.anbehav.2010.01.013.
- ↑ Clarke, P. M. R.; Henzi, S. P.; Barrett, L. (2009). "Sexual conflict in chacma baboons, Papio hamadryas ursinus: absent males select for proactive females". Animal Behaviour. 77: 1217–1225. doi:10.1016/j.anbehav.2009.02.003.
- ↑ Palombit, R. A.; Cheney, D. L.; Seyfarth R. M. (2001). "Female–female competition for male 'friends' in wild chacma baboons, Papio cynocephalus ursinus" (PDF). Animal Behaviour. 61: 1159–1171. doi:10.1006/anbe.2000.1690.
- ↑ Busse, C. (1980). "Leopard and lion predation upon chacma baboons living in the Moremi Wildlife Reserve". Botswana Notes & Records. 12: 15–21.
- ↑ "Darwin Primate Group". Retrieved 9 December 2012.
External links
| Wikispecies has information related to: Chacma baboon |
| Wikimedia Commons has media related to Chacma baboon. |
| Wikisource has the text of the 1911 Encyclopædia Britannica article Chacma. |
- Cape Peninsula Baboon Research Unit
- Darwin Primate Group
- Imfene Baboon Conservation and Education Centre
