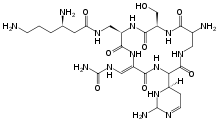
Capreomycin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Capastat |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682860 |
| Pregnancy category |
|
| Routes of administration | intramuscular |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| Chemical and physical data | |
| Formula | C25H44N14O8 |
| Molar mass | 668.706 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Capreomycin is an antibiotic which is given in combination with other antibiotics for the treatment of tuberculosis. Specifically it is a second line treatment used for active drug resistant tuberculosis. It is given by injection into a vein or muscle.[1]
Common side effects include kidney problems, hearing problems, poor balance, and pain at the site of injection. Other side effects include paralysis resulting in the inability to breath. It is not recommended with streptomycin or other medications that may damage the auditory vestibular nerve. It is not recommended during pregnancy as it may cause kidney or hearing problems in the baby.[1] Capreomycin is commonly grouped with the aminoglycoside family of medications.[2] How it works is unclear.[1]
Capreomycin was discovered from Streptomyces capreolus in 1960.[3] It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.[4] The wholesale cost in the developing world is about 6.25 to 8.98 USD a dose.[5]
Spectrum of susceptibility
Capreomycin is most commonly used to treat Mycobacterium tuberculosis infections. Mycobacterium tuberculosis growth has been found to be inhibited at a concentration of 2.5 μg/mL.[6]
Side effects
High incidence: hematuria, urine output or urinary frequency significantly increased or decreased, loss of appetite or extreme thirst (hypokalemia, renal toxicity).
Less incidence: hearing loss, tinnitus, gait instability, dizziness, dyspnea, lethargy, extreme weakness (neuromuscular block, Renal toxicity, hypokalemia), nausea or vomiting.
Significant renal toxicity: creatinine, elevated urea nitrogen, poor creatinine clearance, proteinuria, tubular urine (need monitoring of renal function and urine routine during using this medicine.
Damage on the 8th brain nerve. There can be vestibular dysfunction and a little hearing damage after using the medication for 2 to 4 months.
A certain block effect of neuromuscular.
Can cause allergic reactions: rash, drug fever, facial flushing or pale, asthma, palpitations, sense of suppression in the chest, abdominal pain, anaphylactic shock.
Interactions
Combined with aminoglycosides: can increase the possibility of ototoxicity, nephrotoxicity and neuromuscular block, result in hearing loss. Can continue to deafness. It could be a temporary symptom, but often be permanent. Neuromuscular blockade can lead to skeletal muscle weakness and respiratory depression or paralysis (apnea). Using anti-cholinesterase or calcium salts may release this block.
Combined with amphotericin B, vancomycin, bacitracin, paromomycin, cyclosporine, kanamycin, cisplatin, bumetanide, etoric acid, furosemide: Would Increase the possibility of ototoxicity and nephrotoxicity.
Combined with antihistamines, buclizine, seyclizine, meclozine, phenothiazines, thioketones, trimethamine, and capreomycin: cover up the symptoms of tinnitus, dizziness or vertigo and other ototoxic symptoms.
Combined with anti-neuromuscular block drugs: can antagonize the effect of the anti-neuromuscular block drugs on the skeletal muscle (so need to adjust the dose of the drugs for anti-muscle weakness.
Combined with ethyl sulfide isoniazid: may increase the side effects.
Combined with methoxyflurane or polymyxin injection: may increase renal toxicity or neuromuscular blockade effect.
Combined with opioid: The effect of central respiratory inhibition may increase, lead to prolonged respiratory inhibition or respiratory paralysis (apnea).
History
Capreomycin, an antiphlogistic antibiotic which was produced in USA in 1960, and be applied in clinic in 1968. In 1979, capreomycin was used in the are of antituberculosis by inhibiting the growth of mycobacterium tuberculosis.
References
- 1 2 3 "Capreomycin Sulfate". The American Society of Health-System Pharmacists. Retrieved 8 December 2016.
- ↑ Navneet, Kumar (2015). Textbook of Neurology. PHI Learning Pvt. Ltd. p. 192. ISBN 9788120342439.
- ↑ Tomlinson, Catherine. "TB Online - Capreomycin". Retrieved 14 September 2014.
- ↑ "WHO Model List of Essential Medicines (19th List)" (PDF). World Health Organization. April 2015. Retrieved 8 December 2016.
- ↑ "Capreomycin". International Drug Price Indicator Guide. Retrieved 8 December 2016.
- ↑ http://www.toku-e.com/Assets/MIC/Capreomycin%20sulfate.pdf