Myc
Myc (c-Myc) is a regulator gene that codes for a transcription factor. The protein encoded by this gene is a multifunctional, nuclear phosphoprotein that plays a role in cell cycle progression, apoptosis and cellular transformation.[5]
A mutated version of Myc is found in many cancers, which causes Myc to be constitutively (persistently) expressed. This leads to the unregulated expression of many genes, some of which are involved in cell proliferation, and results in the formation of cancer.[5] A common human translocation involving Myc is critical to the development of most cases of Burkitt lymphoma.[6] Malfunctions in Myc have also been found in carcinoma of the cervix, colon, breast, lung and stomach.[5] Myc is thus viewed as a promising target for anti-cancer drugs.[7]
In the human genome, Myc is located on chromosome 8 and is believed to regulate expression of 15% of all genes[8] through binding on enhancer box sequences (E-boxes) and recruiting histone acetyltransferases (HATs). This means that in addition to its role as a classical transcription factor, Myc also functions to regulate global chromatin structure by regulating histone acetylation both in gene-rich regions and at sites far from any known gene.[9]
Discovery
Myc gene was first discovered in Burkitt lymphoma patients. In Burkitt lymphoma, cancer cells show chromosomal translocations, in which chromosome 8 is frequently involved. Cloning the break-point of the fusion chromosomes revealed a gene that was similar to myelocytomatosis viral oncogene (v-Myc). Thus, the newfound cellular gene was named c-Myc.
Structure
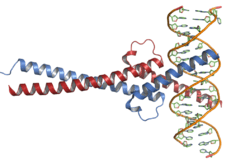
Myc protein belongs to Myc family of transcription factors, which also includes N-Myc and L-Myc genes. Myc family of transcription factors contain a bHLH (basic helix-loop-helix) structural and LZ (leucine zipper) motives. Through its bHLH DNA-binding motif, Myc interacts with DNA, while the leucine zipper TF-binding motif allows the dimerization with its partner Max, another bHLH transcription factor.
Myc mRNA contains an IRES (internal ribosome entry site) that allows the RNA to be translated into protein when 5' cap-dependent translation is inhibited, such as during viral infection.
Function
Myc protein is a transcription factor that activates expression of many genes through binding enhancer box sequences (E-boxes) and recruiting histone acetyltransferases (HATs). It can also act as a transcriptional repressor. By binding Miz-1 transcription factor and displacing the p300 co-activator, it inhibits expression of Miz-1 target genes. In addition, myc has a direct role in the control of DNA replication.[10]
Myc is activated upon various mitogenic signals such as serum stimulation or by Wnt, Shh and EGF (via the MAPK/ERK pathway).[11] By modifying the expression of its target genes, Myc activation results in numerous biological effects. The first to be discovered was its capability to drive cell proliferation (upregulates cyclins, downregulates p21), but it also plays a very important role in regulating cell growth (upregulates ribosomal RNA and proteins), apoptosis (downregulates Bcl-2), differentiation, and stem cell self-renewal. Myc is a very strong proto-oncogene and it is very often found to be upregulated in many types of cancers. Myc overexpression stimulates gene amplification,[12] presumably through DNA over-replication.
There have been several studies that have clearly indicated Myc's role in cell competition.[13]
A major effect of Myc is B cell proliferation.[14]
c-Myc induces MTDH(AEG-1) gene expression and in turn itself requires AEG-1 oncogene for its expression.
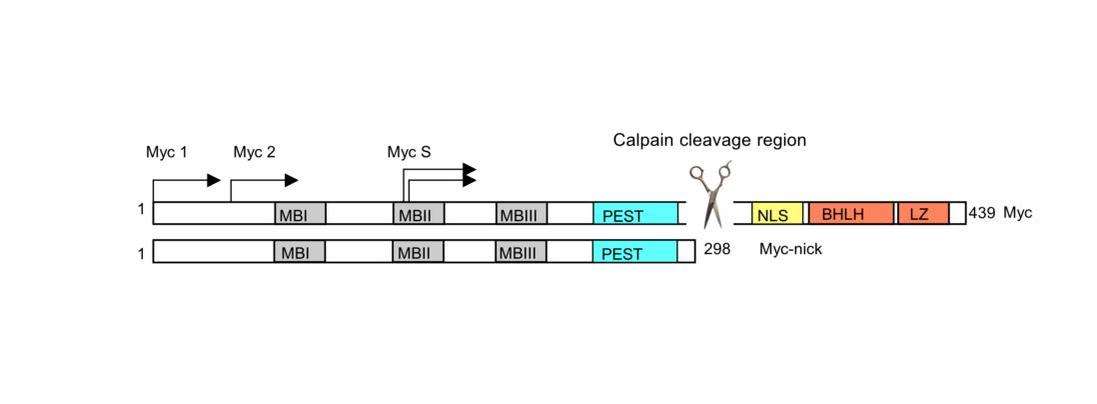
Myc-nick
Myc-nick is a cytoplasmic form of Myc produced by a partial proteolytic cleavage of full-length c-Myc and N-Myc.[15] Myc cleavage is mediated by the calpain family of calcium-dependent cytosolic proteases.
The cleavage of Myc by calpains is a constitutive process but is enhanced under conditions that require rapid downregulation of Myc levels, such as during terminal differentiation. Upon cleavage, the C-terminus of Myc (containing the DNA binding domain) is degraded, while Myc-nick, the N-terminal segment 298-residue segment remains in the cytoplasm. Myc-nick contains binding domains for histone acetyltransferases and for ubiquitin ligases.
The functions of Myc-nick are currently under investigation, but this new Myc family member was found to regulate cell morphology, at least in part, by interacting with acetyl transferases to promote the acetylation of α-tubulin. Ectopic expression of Myc-nick accelerates the differentiation of committed myoblasts into muscle cells.
Clinical significance
Except for early response genes, Myc universally upregulates gene expression. Furthermore, the upregulation is nonlinear. Genes whose expression is already significantly upregulated in the absence of Myc are strongly boosted in the presence of Myc, whereas genes whose expression is low in the absence Myc get only a small boost when Myc is present.[16]
Inactivation of SUMO-activating enzyme (SAE1 / SAE2) in the presence of Myc hyperactivation results in mitotic catastrophe and cell death in cancer cells. Hence inhibitors of SUMOylation may be a possible treatment for cancer.[17]
Amplification of the MYC gene was found in a significant number of epithelial ovarian cancer cases.[18] In TCGA datasets, the amplification of Myc occurs in several cancer types, including breast, colorectal, pancreatic, gastric, and uterine cancers.[19]
In the experimental transformation process of normal cells into cancer cells, the MYC gene can cooperate with the RAS gene.[20][21]
Expression of Myc is highly dependent on BRD4 function in some cancers.[22][23] BET inhibitors have been used to successfully block Myc function in pre-clinical cancer models and are currently being evaluated in clinical trials.[24][25]
Animal Models
During the discovery of Myc gene, it was realized that chromosomes that reciprocally translocate to chromosome 8 contained immunoglobulin genes at the break-point. Enhancers that normally drive expression of immunoglobin genes now lead to overexpression of Myc proto-oncogene in lymphoma cells. To study the mechanism of tumorigenesis in Burkitt lymphoma by mimicking expression pattern of Myc in these cancer cells, transgenic mouse models were developed. Myc gene placed under the control of IgM heavy chain enhancer in transgenic mice gives rise to mainly lymphomas. Later on, in order to study effects of Myc in other types of cancer, transgenic mice that overexpress Myc in different tissues (liver, breast) were also made. In all these mouse models overexpression of Myc causes tumorigenesis, illustrating the potency of Myc oncogene. In a study with mice, reduced expression of Myc was shown to induce longevity, with significantly extended median and maximum lifespans in both sexes and a reduced mortality rate across all ages, better health, cancer progression was slower, better metabolism and they had smaller bodies. Also, Less TOR, AKT, S6K and other changes in energy and metabolic pathways (such as AMPK, more oxygen consumption, more body movements, etc). The study by John M. Sedivy and others used Cre-Loxp -recombinase to knockout one copy of Myc and this resulted in a "Haplo-insufficient" genotype noted as Myc+/-. The phenotypes seen oppose the effects of normal aging and are shared with many other long-lived mouse models such as CR (calorie restriction) ames dwarf, rapamycin, metformin and resveratrol. One study found that Myc and p53 genes were key to the survival of Chronic Myeloid Leukaemia (CML) cells. Targeting Myc and p53 proteins with drugs gave positive results on mice with CML.[26][27]
Use in biology
C-myc plays a major role in the generation of Induced pluripotent stem cell (iPS). It is one of the four Yamanaka's factor (along with three others transcription factors : Oct4, Sox2 and Klf4). Even though it has since been possible to generate iPS without c-MYC.
Interactions
Myc has been shown to interact with:
- ACTL6A[28]
- BRCA1[29][30][31][32]
- Bcl-2[33]
- Cyclin T1[34]
- CHD8[35]
- DNMT3A[36]
- EP400[37]
- GTF2I[38]
- HTATIP[39]
- let-7[40][41][42]
- MAPK1[33][43][44]
- MAPK8[45]
- MAX[46][47][48][49][50][51][52][53][54][55][56][57][58]
- MLH1[50]
- MYCBP2[59]
- MYCBP[60]
- NMI[29]
- NFYB[61]
- NFYC[62]
- P73[63]
- PCAF[64]
- PFDN5[65][66]
- RuvB-like 1[28][37]
- SAP130[64]
- SMAD2[67]
- SMAD3[67]
- SMARCA4[28][46]
- SMARCB1[49]
- SUPT3H[64]
- TIAM1[68]
- TADA2L[64]
- TAF9[64]
- TFAP2A[69]
- TRRAP[28][47][48][64]
- WDR5[70]
- YY1[71] and
- ZBTB17.[72][73]

See also
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000136997 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000022346 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- 1 2 3 "Myc". NCBI.
- ↑ Finver SN, Nishikura K, Finger LR, Haluska FG, Finan J, Nowell PC, Croce CM (May 1988). "Sequence analysis of the MYC oncogene involved in the t(8;14)(q24;q11) chromosome translocation in a human leukemia T-cell line indicates that putative regulatory regions are not altered". Proceedings of the National Academy of Sciences of the United States of America. 85 (9): 3052–6. PMC 280141
 . PMID 2834731. doi:10.1073/pnas.85.9.3052.
. PMID 2834731. doi:10.1073/pnas.85.9.3052. - ↑ Begley S (2013-01-09). "DNA pioneer James Watson takes aim at cancer establishments". Reuters.
- ↑ Gearhart J, Pashos EE, Prasad MK (October 2007). "Pluripotency redux--advances in stem-cell research". The New England Journal of Medicine. 357 (15): 1469–72. PMID 17928593. doi:10.1056/NEJMp078126.
- ↑ Cotterman R, Jin VX, Krig SR, Lemen JM, Wey A, Farnham PJ, Knoepfler PS (December 2008). "N-Myc regulates a widespread euchromatic program in the human genome partially independent of its role as a classical transcription factor". Cancer Research. 68 (23): 9654–62. PMC 2637654
 . PMID 19047142. doi:10.1158/0008-5472.CAN-08-1961.
. PMID 19047142. doi:10.1158/0008-5472.CAN-08-1961. - ↑ Dominguez-Sola D, Ying CY, Grandori C, Ruggiero L, Chen B, Li M, Galloway DA, Gu W, Gautier J, Dalla-Favera R (July 2007). "Non-transcriptional control of DNA replication by c-Myc". Nature. 448 (7152): 445–51. PMID 17597761. doi:10.1038/nature05953.
- ↑ Campisi J, Gray HE, Pardee AB, Dean M, Sonenshein GE (1984). "Cell-cycle control of c-myc but not c-ras expression is lost following chemical transformation". Cell. 36 (2): 241–7. PMID 6692471. doi:10.1016/0092-8674(84)90217-4.
- ↑ Denis N, Kitzis A, Kruh J, Dautry F, Corcos D (August 1991). "Stimulation of methotrexate resistance and dihydrofolate reductase gene amplification by c-myc". Oncogene. 6 (8): 1453–7. PMID 1886715.
- ↑ Clavería C, Giovinazzo G, Sierra R, Torres M (August 2013). "Myc-driven endogenous cell competition in the early mammalian embryo". Nature. 500 (7460): 39–44. PMID 23842495. doi:10.1038/nature12389.
- ↑ de Alboran IM, O'Hagan RC, Gärtner F, Malynn B, Davidson L, Rickert R, Rajewsky K, DePinho RA, Alt FW (January 2001). "Analysis of C-MYC function in normal cells via conditional gene-targeted mutation". Immunity. 14 (1): 45–55. PMID 11163229. doi:10.1016/S1074-7613(01)00088-7.
- ↑ Conacci-Sorrell M, Ngouenet C, Eisenman RN (August 2010). "Myc-nick: a cytoplasmic cleavage product of Myc that promotes alpha-tubulin acetylation and cell differentiation". Cell. 142 (3): 480–93. PMC 2923036
 . PMID 20691906. doi:10.1016/j.cell.2010.06.037.
. PMID 20691906. doi:10.1016/j.cell.2010.06.037. - ↑ Nie Z, Hu G, Wei G, Cui K, Yamane A, Resch W, Wang R, Green DR, Tessarollo L, Casellas R, Zhao K, Levens D (September 2012). "c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells". Cell. 151 (1): 68–79. PMC 3471363
 . PMID 23021216. doi:10.1016/j.cell.2012.08.033.
. PMID 23021216. doi:10.1016/j.cell.2012.08.033. - ↑ Kessler JD, Kahle KT, Sun T, Meerbrey KL, Schlabach MR, Schmitt EM, Skinner SO, Xu Q, Li MZ, Hartman ZC, Rao M, Yu P, Dominguez-Vidana R, Liang AC, Solimini NL, Bernardi RJ, Yu B, Hsu T, Golding I, Luo J, Osborne CK, Creighton CJ, Hilsenbeck SG, Schiff R, Shaw CA, Elledge SJ, Westbrook TF (January 2012). "A SUMOylation-dependent transcriptional subprogram is required for Myc-driven tumorigenesis". Science. 335 (6066): 348–53. PMC 4059214
 . PMID 22157079. doi:10.1126/science.1212728.
. PMID 22157079. doi:10.1126/science.1212728. - ↑ Ross JS, Ali SM, Wang K, Palmer G, Yelensky R, Lipson D, Miller VA, Zajchowski D, Shawver LK, Stephens PJ (September 2013). "Comprehensive genomic profiling of epithelial ovarian cancer by next generation sequencing-based diagnostic assay reveals new routes to targeted therapies". Gynecologic Oncology. 130 (3): 554–9. PMID 23791828. doi:10.1016/j.ygyno.2013.06.019.
- ↑ Chen Y, McGee J, Chen X, Doman TN, Gong X, Zhang Y, Hamm N, Ma X, Higgs RE, Bhagwat SV, Buchanan S, Peng SB, Staschke KA, Yadav V, Yue Y, Kouros-Mehr H (2014). "Identification of druggable cancer driver genes amplified across TCGA datasets". PloS One. 9 (5): e98293. PMC 4038530
 . PMID 24874471. doi:10.1371/journal.pone.0098293.
. PMID 24874471. doi:10.1371/journal.pone.0098293. - ↑ Land H, Parada LF, Weinberg RA (1983). "Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes". Nature. 304 (5927): 596–602. PMID 6308472. doi:10.1038/304596a0.
- ↑ Radner H, el-Shabrawi Y, Eibl RH, Brüstle O, Kenner L, Kleihues P, Wiestler OD (1993). "Tumor induction by ras and myc oncogenes in fetal and neonatal brain: modulating effects of developmental stage and retroviral dose". Acta Neuropathologica. 86 (5): 456–65. PMID 8310796. doi:10.1007/bf00228580.
- ↑ Fowler T, Ghatak P, Price DH, Conaway R, Conaway J, Chiang CM, Bradner JE, Shilatifard A, Roy AL (2014). "Regulation of MYC expression and differential JQ1 sensitivity in cancer cells". PloS One. 9 (1): e87003. PMC 3900694
 . PMID 24466310. doi:10.1371/journal.pone.0087003.
. PMID 24466310. doi:10.1371/journal.pone.0087003. - ↑ Shi J, Vakoc CR (June 2014). "The mechanisms behind the therapeutic activity of BET bromodomain inhibition". Molecular Cell. 54 (5): 728–36. PMC 4236231
 . PMID 24905006. doi:10.1016/j.molcel.2014.05.016.
. PMID 24905006. doi:10.1016/j.molcel.2014.05.016. - ↑ Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM, Kastritis E, Gilpatrick T, Paranal RM, Qi J, Chesi M, Schinzel AC, McKeown MR, Heffernan TP, Vakoc CR, Bergsagel PL, Ghobrial IM, Richardson PG, Young RA, Hahn WC, Anderson KC, Kung AL, Bradner JE, Mitsiades CS (September 2011). "BET bromodomain inhibition as a therapeutic strategy to target c-Myc". Cell. 146 (6): 904–17. PMC 3187920
 . PMID 21889194. doi:10.1016/j.cell.2011.08.017.
. PMID 21889194. doi:10.1016/j.cell.2011.08.017. - ↑ Fu LL, Tian M, Li X, Li JJ, Huang J, Ouyang L, Zhang Y, Liu B (March 2015). "Inhibition of BET bromodomains as a therapeutic strategy for cancer drug discovery". Oncotarget. 6 (8): 5501–16. PMC 4467383
 . PMID 25849938. doi:10.18632/oncotarget.3551.
. PMID 25849938. doi:10.18632/oncotarget.3551. - ↑ Abraham SA, Hopcroft LE, Carrick E, Drotar ME, Dunn K, Williamson AJ, Korfi K, Baquero P, Park LE, Scott MT, Pellicano F, Pierce A, Copland M, Nourse C, Grimmond SM, Vetrie D, Whetton AD, Holyoake TL (June 2016). "Dual targeting of p53 and c-MYC selectively eliminates leukaemic stem cells". Nature. 534 (7607): 341–6. PMC 4913876
 . PMID 27281222. doi:10.1038/nature18288.
. PMID 27281222. doi:10.1038/nature18288. - ↑ "Scientists identify drugs to target ’Achilles heel’ of Chronic Myeloid Leukaemia cells". myScience. 2016-06-08. Retrieved 2016-06-09.
- 1 2 3 4 Park J, Wood MA, Cole MD (March 2002). "BAF53 forms distinct nuclear complexes and functions as a critical c-Myc-interacting nuclear cofactor for oncogenic transformation". Molecular and Cellular Biology. 22 (5): 1307–16. PMC 134713
 . PMID 11839798. doi:10.1128/mcb.22.5.1307-1316.2002.
. PMID 11839798. doi:10.1128/mcb.22.5.1307-1316.2002. - 1 2 Li H, Lee TH, Avraham H (June 2002). "A novel tricomplex of BRCA1, Nmi, and c-Myc inhibits c-Myc-induced human telomerase reverse transcriptase gene (hTERT) promoter activity in breast cancer". The Journal of Biological Chemistry. 277 (23): 20965–73. PMID 11916966. doi:10.1074/jbc.M112231200.
- ↑ Xiong J, Fan S, Meng Q, Schramm L, Wang C, Bouzahza B, Zhou J, Zafonte B, Goldberg ID, Haddad BR, Pestell RG, Rosen EM (December 2003). "BRCA1 inhibition of telomerase activity in cultured cells". Molecular and Cellular Biology. 23 (23): 8668–90. PMC 262673
 . PMID 14612409. doi:10.1128/mcb.23.23.8668-8690.2003.
. PMID 14612409. doi:10.1128/mcb.23.23.8668-8690.2003. - ↑ Zhou C, Liu J (March 2003). "Inhibition of human telomerase reverse transcriptase gene expression by BRCA1 in human ovarian cancer cells". Biochemical and Biophysical Research Communications. 303 (1): 130–6. PMID 12646176. doi:10.1016/s0006-291x(03)00318-8.
- ↑ Wang Q, Zhang H, Kajino K, Greene MI (October 1998). "BRCA1 binds c-Myc and inhibits its transcriptional and transforming activity in cells". Oncogene. 17 (15): 1939–48. PMID 9788437. doi:10.1038/sj.onc.1202403.
- 1 2 Jin Z, Gao F, Flagg T, Deng X (September 2004). "Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone promotes functional cooperation of Bcl2 and c-Myc through phosphorylation in regulating cell survival and proliferation". The Journal of Biological Chemistry. 279 (38): 40209–19. PMID 15210690. doi:10.1074/jbc.M404056200.
- ↑ Kanazawa S, Soucek L, Evan G, Okamoto T, Peterlin BM (August 2003). "c-Myc recruits P-TEFb for transcription, cellular proliferation and apoptosis". Oncogene. 22 (36): 5707–11. PMID 12944920. doi:10.1038/sj.onc.1206800.
- ↑ Dingar D, Kalkat M, Chan PK, Srikumar T, Bailey SD, Tu WB, Coyaud E, Ponzielli R, Kolyar M, Jurisica I, Huang A, Lupien M, Penn LZ, Raught B (April 2015). "BioID identifies novel c-MYC interacting partners in cultured cells and xenograft tumors". Journal of Proteomics. 118 (12): 95–111. PMID 25452129. doi:10.1016/j.jprot.2014.09.029.
- ↑ Brenner C, Deplus R, Didelot C, Loriot A, Viré E, De Smet C, Gutierrez A, Danovi D, Bernard D, Boon T, Pelicci PG, Amati B, Kouzarides T, de Launoit Y, Di Croce L, Fuks F (January 2005). "Myc represses transcription through recruitment of DNA methyltransferase corepressor". The EMBO Journal. 24 (2): 336–46. PMC 545804
 . PMID 15616584. doi:10.1038/sj.emboj.7600509.
. PMID 15616584. doi:10.1038/sj.emboj.7600509. - 1 2 Fuchs M, Gerber J, Drapkin R, Sif S, Ikura T, Ogryzko V, Lane WS, Nakatani Y, Livingston DM (August 2001). "The p400 complex is an essential E1A transformation target". Cell. 106 (3): 297–307. PMID 11509179. doi:10.1016/s0092-8674(01)00450-0.
- ↑ Roy AL, Carruthers C, Gutjahr T, Roeder RG (September 1993). "Direct role for Myc in transcription initiation mediated by interactions with TFII-I". Nature. 365 (6444): 359–61. PMID 8377829. doi:10.1038/365359a0.
- ↑ Frank SR, Parisi T, Taubert S, Fernandez P, Fuchs M, Chan HM, Livingston DM, Amati B (June 2003). "MYC recruits the TIP60 histone acetyltransferase complex to chromatin". EMBO Reports. 4 (6): 575–80. PMC 1319201
 . PMID 12776177. doi:10.1038/sj.embor.embor861.
. PMID 12776177. doi:10.1038/sj.embor.embor861. - ↑ Chang TC, Yu D, Lee YS, Wentzel EA, Arking DE, West KM, Dang CV, Thomas-Tikhonenko A, Mendell JT (January 2008). "Widespread microRNA repression by Myc contributes to tumorigenesis". Nature Genetics. 40 (1): 43–50. PMC 2628762
 . PMID 18066065. doi:10.1038/ng.2007.30.
. PMID 18066065. doi:10.1038/ng.2007.30. - ↑ Koscianska E, Baev V, Skreka K, Oikonomaki K, Rusinov V, Tabler M, Kalantidis K (2007). "Prediction and preliminary validation of oncogene regulation by miRNAs". BMC Molecular Biology. 8: 79. PMC 2096627
 . PMID 17877811. doi:10.1186/1471-2199-8-79.
. PMID 17877811. doi:10.1186/1471-2199-8-79. - ↑ Ioannidis P, Mahaira LG, Perez SA, Gritzapis AD, Sotiropoulou PA, Kavalakis GJ, Antsaklis AI, Baxevanis CN, Papamichail M (May 2005). "CRD-BP/IMP1 expression characterizes cord blood CD34+ stem cells and affects c-myc and IGF-II expression in MCF-7 cancer cells". The Journal of Biological Chemistry. 280 (20): 20086–93. PMID 15769738. doi:10.1074/jbc.M410036200.
- ↑ Gupta S, Davis RJ (October 1994). "MAP kinase binds to the NH2-terminal activation domain of c-Myc". FEBS Letters. 353 (3): 281–5. PMID 7957875. doi:10.1016/0014-5793(94)01052-8.
- ↑ Tournier C, Whitmarsh AJ, Cavanagh J, Barrett T, Davis RJ (July 1997). "Mitogen-activated protein kinase kinase 7 is an activator of the c-Jun NH2-terminal kinase". Proceedings of the National Academy of Sciences of the United States of America. 94 (14): 7337–42. PMC 23822
 . PMID 9207092. doi:10.1073/pnas.94.14.7337.
. PMID 9207092. doi:10.1073/pnas.94.14.7337. - ↑ Noguchi K, Kitanaka C, Yamana H, Kokubu A, Mochizuki T, Kuchino Y (November 1999). "Regulation of c-Myc through phosphorylation at Ser-62 and Ser-71 by c-Jun N-terminal kinase". The Journal of Biological Chemistry. 274 (46): 32580–7. PMID 10551811. doi:10.1074/jbc.274.46.32580.
- 1 2 Ewing RM, Chu P, Elisma F, Li H, Taylor P, Climie S, McBroom-Cerajewski L, Robinson MD, O'Connor L, Li M, Taylor R, Dharsee M, Ho Y, Heilbut A, Moore L, Zhang S, Ornatsky O, Bukhman YV, Ethier M, Sheng Y, Vasilescu J, Abu-Farha M, Lambert JP, Duewel HS, Stewart II, Kuehl B, Hogue K, Colwill K, Gladwish K, Muskat B, Kinach R, Adams SL, Moran MF, Morin GB, Topaloglou T, Figeys D (2007). "Large-scale mapping of human protein-protein interactions by mass spectrometry". Molecular Systems Biology. 3: 89. PMC 1847948
 . PMID 17353931. doi:10.1038/msb4100134.
. PMID 17353931. doi:10.1038/msb4100134. - 1 2 McMahon SB, Wood MA, Cole MD (January 2000). "The essential cofactor TRRAP recruits the histone acetyltransferase hGCN5 to c-Myc". Molecular and Cellular Biology. 20 (2): 556–62. PMC 85131
 . PMID 10611234. doi:10.1128/mcb.20.2.556-562.2000.
. PMID 10611234. doi:10.1128/mcb.20.2.556-562.2000. - 1 2 McMahon SB, Van Buskirk HA, Dugan KA, Copeland TD, Cole MD (August 1998). "The novel ATM-related protein TRRAP is an essential cofactor for the c-Myc and E2F oncoproteins". Cell. 94 (3): 363–74. PMID 9708738. doi:10.1016/s0092-8674(00)81479-8.
- 1 2 Cheng SW, Davies KP, Yung E, Beltran RJ, Yu J, Kalpana GV (May 1999). "c-MYC interacts with INI1/hSNF5 and requires the SWI/SNF complex for transactivation function". Nature Genetics. 22 (1): 102–5. PMID 10319872. doi:10.1038/8811.
- 1 2 Mac Partlin M, Homer E, Robinson H, McCormick CJ, Crouch DH, Durant ST, Matheson EC, Hall AG, Gillespie DA, Brown R (February 2003). "Interactions of the DNA mismatch repair proteins MLH1 and MSH2 with c-MYC and MAX". Oncogene. 22 (6): 819–25. PMID 12584560. doi:10.1038/sj.onc.1206252.
- ↑ Blackwood EM, Eisenman RN (March 1991). "Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc". Science. 251 (4998): 1211–7. PMID 2006410. doi:10.1126/science.2006410.
- ↑ Lee CM, Onésime D, Reddy CD, Dhanasekaran N, Reddy EP (October 2002). "JLP: A scaffolding protein that tethers JNK/p38MAPK signaling modules and transcription factors". Proceedings of the National Academy of Sciences of the United States of America. 99 (22): 14189–94. PMC 137859
 . PMID 12391307. doi:10.1073/pnas.232310199.
. PMID 12391307. doi:10.1073/pnas.232310199. - ↑ Billin AN, Eilers AL, Queva C, Ayer DE (December 1999). "Mlx, a novel Max-like BHLHZip protein that interacts with the Max network of transcription factors". The Journal of Biological Chemistry. 274 (51): 36344–50. PMID 10593926. doi:10.1074/jbc.274.51.36344.
- ↑ Gupta K, Anand G, Yin X, Grove L, Prochownik EV (March 1998). "Mmip1: a novel leucine zipper protein that reverses the suppressive effects of Mad family members on c-myc". Oncogene. 16 (9): 1149–59. PMID 9528857. doi:10.1038/sj.onc.1201634.
- ↑ Meroni G, Reymond A, Alcalay M, Borsani G, Tanigami A, Tonlorenzi R, Lo Nigro C, Messali S, Zollo M, Ledbetter DH, Brent R, Ballabio A, Carrozzo R (May 1997). "Rox, a novel bHLHZip protein expressed in quiescent cells that heterodimerizes with Max, binds a non-canonical E box and acts as a transcriptional repressor". The EMBO Journal. 16 (10): 2892–906. PMC 1169897
 . PMID 9184233. doi:10.1093/emboj/16.10.2892.
. PMID 9184233. doi:10.1093/emboj/16.10.2892. - ↑ Nair SK, Burley SK (January 2003). "X-ray structures of Myc-Max and Mad-Max recognizing DNA. Molecular bases of regulation by proto-oncogenic transcription factors". Cell. 112 (2): 193–205. PMID 12553908. doi:10.1016/s0092-8674(02)01284-9.
- ↑ FitzGerald MJ, Arsura M, Bellas RE, Yang W, Wu M, Chin L, Mann KK, DePinho RA, Sonenshein GE (April 1999). "Differential effects of the widely expressed dMax splice variant of Max on E-box vs initiator element-mediated regulation by c-Myc". Oncogene. 18 (15): 2489–98. PMID 10229200. doi:10.1038/sj.onc.1202611.
- ↑ Meroni G, Cairo S, Merla G, Messali S, Brent R, Ballabio A, Reymond A (July 2000). "Mlx, a new Max-like bHLHZip family member: the center stage of a novel transcription factors regulatory pathway?". Oncogene. 19 (29): 3266–77. PMID 10918583. doi:10.1038/sj.onc.1203634.
- ↑ Guo Q, Xie J, Dang CV, Liu ET, Bishop JM (August 1998). "Identification of a large Myc-binding protein that contains RCC1-like repeats". Proceedings of the National Academy of Sciences of the United States of America. 95 (16): 9172–7. PMC 21311
 . PMID 9689053. doi:10.1073/pnas.95.16.9172.
. PMID 9689053. doi:10.1073/pnas.95.16.9172. - ↑ Taira T, Maëda J, Onishi T, Kitaura H, Yoshida S, Kato H, Ikeda M, Tamai K, Iguchi-Ariga SM, Ariga H (August 1998). "AMY-1, a novel C-MYC binding protein that stimulates transcription activity of C-MYC". Genes to Cells. 3 (8): 549–65. PMID 9797456. doi:10.1046/j.1365-2443.1998.00206.x.
- ↑ Izumi H, Molander C, Penn LZ, Ishisaki A, Kohno K, Funa K (April 2001). "Mechanism for the transcriptional repression by c-Myc on PDGF beta-receptor". Journal of Cell Science. 114 (Pt 8): 1533–44. PMID 11282029.
- ↑ Taira T, Sawai M, Ikeda M, Tamai K, Iguchi-Ariga SM, Ariga H (August 1999). "Cell cycle-dependent switch of up-and down-regulation of human hsp70 gene expression by interaction between c-Myc and CBF/NF-Y". The Journal of Biological Chemistry. 274 (34): 24270–9. PMID 10446203. doi:10.1074/jbc.274.34.24270.
- ↑ Uramoto H, Izumi H, Ise T, Tada M, Uchiumi T, Kuwano M, Yasumoto K, Funa K, Kohno K (August 2002). "p73 Interacts with c-Myc to regulate Y-box-binding protein-1 expression". The Journal of Biological Chemistry. 277 (35): 31694–702. PMID 12080043. doi:10.1074/jbc.M200266200.
- 1 2 3 4 5 6 Liu X, Tesfai J, Evrard YA, Dent SY, Martinez E (May 2003). "c-Myc transformation domain recruits the human STAGA complex and requires TRRAP and GCN5 acetylase activity for transcription activation". The Journal of Biological Chemistry. 278 (22): 20405–12. PMC 4031917
 . PMID 12660246. doi:10.1074/jbc.M211795200.
. PMID 12660246. doi:10.1074/jbc.M211795200. - ↑ Mori K, Maeda Y, Kitaura H, Taira T, Iguchi-Ariga SM, Ariga H (November 1998). "MM-1, a novel c-Myc-associating protein that represses transcriptional activity of c-Myc". The Journal of Biological Chemistry. 273 (45): 29794–800. PMID 9792694. doi:10.1074/jbc.273.45.29794.
- ↑ Fujioka Y, Taira T, Maeda Y, Tanaka S, Nishihara H, Iguchi-Ariga SM, Nagashima K, Ariga H (November 2001). "MM-1, a c-Myc-binding protein, is a candidate for a tumor suppressor in leukemia/lymphoma and tongue cancer". The Journal of Biological Chemistry. 276 (48): 45137–44. PMID 11567024. doi:10.1074/jbc.M106127200.
- 1 2 Feng XH, Liang YY, Liang M, Zhai W, Lin X (January 2002). "Direct interaction of c-Myc with Smad2 and Smad3 to inhibit TGF-beta-mediated induction of the CDK inhibitor p15(Ink4B)". Molecular Cell. 9 (1): 133–43. PMID 11804592. doi:10.1016/s1097-2765(01)00430-0.
- ↑ Otsuki Y, Tanaka M, Kamo T, Kitanaka C, Kuchino Y, Sugimura H (February 2003). "Guanine nucleotide exchange factor, Tiam1, directly binds to c-Myc and interferes with c-Myc-mediated apoptosis in rat-1 fibroblasts". The Journal of Biological Chemistry. 278 (7): 5132–40. PMID 12446731. doi:10.1074/jbc.M206733200.
- ↑ Gaubatz S, Imhof A, Dosch R, Werner O, Mitchell P, Buettner R, Eilers M (April 1995). "Transcriptional activation by Myc is under negative control by the transcription factor AP-2". The EMBO Journal. 14 (7): 1508–19. PMC 398238
 . PMID 7729426.
. PMID 7729426. - ↑ Thomas LR, Wang Q, Grieb BC, Phan J, Foshage AM, Sun Q, Olejniczak ET, Clark T, Dey S, Lorey S, Alicie B, Howard GC, Cawthon B, Ess KC, Eischen CM, Zhao Z, Fesik SW, Tansey WP (May 2015). "Interaction with WDR5 promotes target gene recognition and tumorigenesis by MYC". Molecular Cell. 58 (3): 440–52. PMC 4427524
 . PMID 25818646. doi:10.1016/j.molcel.2015.02.028.
. PMID 25818646. doi:10.1016/j.molcel.2015.02.028. - ↑ Shrivastava A, Saleque S, Kalpana GV, Artandi S, Goff SP, Calame K (December 1993). "Inhibition of transcriptional regulator Yin-Yang-1 by association with c-Myc". Science. 262 (5141): 1889–92. PMID 8266081. doi:10.1126/science.8266081.
- ↑ Staller P, Peukert K, Kiermaier A, Seoane J, Lukas J, Karsunky H, Möröy T, Bartek J, Massagué J, Hänel F, Eilers M (April 2001). "Repression of p15INK4b expression by Myc through association with Miz-1". Nature Cell Biology. 3 (4): 392–9. PMID 11283613. doi:10.1038/35070076.
- ↑ Peukert K, Staller P, Schneider A, Carmichael G, Hänel F, Eilers M (September 1997). "An alternative pathway for gene regulation by Myc". The EMBO Journal. 16 (18): 5672–86. PMC 1170199
 . PMID 9312026. doi:10.1093/emboj/16.18.5672.
. PMID 9312026. doi:10.1093/emboj/16.18.5672.
Further reading
- Ruf IK, Rhyne PW, Yang H, Borza CM, Hutt-Fletcher LM, Cleveland JL, Sample JT (2001). "EBV regulates c-MYC, apoptosis, and tumorigenicity in Burkitt's lymphoma". Current Topics in Microbiology and Immunology. 258: 153–60. PMID 11443860.
- Lüscher B (October 2001). "Function and regulation of the transcription factors of the Myc/Max/Mad network". Gene. 277 (1–2): 1–14. PMID 11602341. doi:10.1016/S0378-1119(01)00697-7.
- Hoffman B, Amanullah A, Shafarenko M, Liebermann DA (May 2002). "The proto-oncogene c-myc in hematopoietic development and leukemogenesis". Oncogene. 21 (21): 3414–21. PMID 12032779. doi:10.1038/sj.onc.1205400.
- Pelengaris S, Khan M, Evan G (October 2002). "c-MYC: more than just a matter of life and death". Nature Reviews. Cancer. 2 (10): 764–76. PMID 12360279. doi:10.1038/nrc904.
- Nilsson JA, Cleveland JL (December 2003). "Myc pathways provoking cell suicide and cancer". Oncogene. 22 (56): 9007–21. PMID 14663479. doi:10.1038/sj.onc.1207261.
- Dang CV, O'donnell KA, Juopperi T (September 2005). "The great MYC escape in tumorigenesis". Cancer Cell. 8 (3): 177–8. PMID 16169462. doi:10.1016/j.ccr.2005.08.005.
- Dang CV, Li F, Lee LA (November 2005). "Could MYC induction of mitochondrial biogenesis be linked to ROS production and genomic instability?". Cell Cycle. 4 (11): 1465–6. PMID 16205115. doi:10.4161/cc.4.11.2121.
- Coller HA, Forman JJ, Legesse-Miller A (August 2007). ""Myc'ed messages": myc induces transcription of E2F1 while inhibiting its translation via a microRNA polycistron". PLoS Genetics. 3 (8): e146. PMC 1959363
 . PMID 17784791. doi:10.1371/journal.pgen.0030146.
. PMID 17784791. doi:10.1371/journal.pgen.0030146. - Astrin SM, Laurence J (May 1992). "Human immunodeficiency virus activates c-myc and Epstein-Barr virus in human B lymphocytes". Annals of the New York Academy of Sciences. 651: 422–32. PMID 1318011. doi:10.1111/j.1749-6632.1992.tb24642.x.
- Bernstein PL, Herrick DJ, Prokipcak RD, Ross J (April 1992). "Control of c-myc mRNA half-life in vitro by a protein capable of binding to a coding region stability determinant". Genes & Development. 6 (4): 642–54. PMID 1559612. doi:10.1101/gad.6.4.642.
- Iijima S, Teraoka H, Date T, Tsukada K (June 1992). "DNA-activated protein kinase in Raji Burkitt's lymphoma cells. Phosphorylation of c-Myc oncoprotein". European Journal of Biochemistry / FEBS. 206 (2): 595–603. PMID 1597196. doi:10.1111/j.1432-1033.1992.tb16964.x.
- Seth A, Alvarez E, Gupta S, Davis RJ (December 1991). "A phosphorylation site located in the NH2-terminal domain of c-Myc increases transactivation of gene expression". The Journal of Biological Chemistry. 266 (35): 23521–4. PMID 1748630.
- Takahashi E, Hori T, O'Connell P, Leppert M, White R (1991). "Mapping of the MYC gene to band 8q24.12----q24.13 by R-banding and distal to fra(8)(q24.11), FRA8E, by fluorescence in situ hybridization". Cytogenetics and Cell Genetics. 57 (2–3): 109–11. PMID 1914517. doi:10.1159/000133124.
- Blackwood EM, Eisenman RN (March 1991). "Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc". Science. 251 (4998): 1211–7. PMID 2006410. doi:10.1126/science.2006410.
- Gazin C, Rigolet M, Briand JP, Van Regenmortel MH, Galibert F (September 1986). "Immunochemical detection of proteins related to the human c-myc exon 1". The EMBO Journal. 5 (9): 2241–50. PMC 1167107
 . PMID 2430795.
. PMID 2430795. - Lüscher B, Kuenzel EA, Krebs EG, Eisenman RN (April 1989). "Myc oncoproteins are phosphorylated by casein kinase II". The EMBO Journal. 8 (4): 1111–9. PMC 400922
 . PMID 2663470.
. PMID 2663470. - Finver SN, Nishikura K, Finger LR, Haluska FG, Finan J, Nowell PC, Croce CM (May 1988). "Sequence analysis of the MYC oncogene involved in the t(8;14)(q24;q11) chromosome translocation in a human leukemia T-cell line indicates that putative regulatory regions are not altered". Proceedings of the National Academy of Sciences of the United States of America. 85 (9): 3052–6. PMC 280141
 . PMID 2834731. doi:10.1073/pnas.85.9.3052.
. PMID 2834731. doi:10.1073/pnas.85.9.3052. - Showe LC, Moore RC, Erikson J, Croce CM (May 1987). "MYC oncogene involved in a t(8;22) chromosome translocation is not altered in its putative regulatory regions". Proceedings of the National Academy of Sciences of the United States of America. 84 (9): 2824–8. PMC 304752
 . PMID 3033665. doi:10.1073/pnas.84.9.2824.
. PMID 3033665. doi:10.1073/pnas.84.9.2824. - Guilhot S, Petridou B, Syed-Hussain S, Galibert F (December 1988). "Nucleotide sequence 3' to the human c-myc oncogene; presence of a long inverted repeat". Gene. 72 (1–2): 105–8. PMID 3243428. doi:10.1016/0378-1119(88)90131-X.
- Hann SR, King MW, Bentley DL, Anderson CW, Eisenman RN (January 1988). "A non-AUG translational initiation in c-myc exon 1 generates an N-terminally distinct protein whose synthesis is disrupted in Burkitt's lymphomas". Cell. 52 (2): 185–95. PMID 3277717. doi:10.1016/0092-8674(88)90507-7.
External links
- The Myc Protein
- NCBI Human Myc protein
- Myc cancer gene
- myc Proto-Oncogene Proteins at the US National Library of Medicine Medical Subject Headings (MeSH)
- Generating iPS Cells from MEFS through Forced Expression of Sox-2, Oct-4, c-Myc, and Klf4
- Drosophila Myc - The Interactive Fly
- FactorBook C-Myc