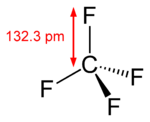
Tetrafluoromethane
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
Tetrafluoromethane Carbon tetrafluoride | |||
| Other names
Carbon tetrafluoride, Perfluoromethane, Tetrafluorocarbon, Freon 14, Halon 14, Arcton 0, CFC 14, PFC 14, R 14, UN 1982 | |||
| Identifiers | |||
| 3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.815 | ||
| EC Number | 200-896-5 | ||
| PubChem CID |
|||
| RTECS number | FG4920000 | ||
| UNII | |||
| |||
| |||
| Properties | |||
| CF4 | |||
| Molar mass | 88.0043 g/mol | ||
| Appearance | Colorless gas | ||
| Odor | odorless | ||
| Density | 3.72 g/l, gas (15 °C) | ||
| Melting point | −183.6 °C (−298.5 °F; 89.5 K) | ||
| Boiling point | −127.8 °C (−198.0 °F; 145.3 K) | ||
| 0.005%V at 20 °C 0.0038%V at 25 °C | |||
| Solubility | soluble in benzene, chloroform | ||
| Vapor pressure | 3.65 MPa at 15 °C 106.5 kPa at −127 °C | ||
| Henry's law constant (kH) |
5.15 atm-cu m/mole | ||
| Refractive index (nD) |
1.0004823 | ||
| Structure | |||
| Tetragonal | |||
| Tetrahedral | |||
| 0 D | |||
| Hazards | |||
| Safety data sheet | ICSC 0575 | ||
| NFPA 704 | |||
| Flash point | Non-flammable | ||
| 1,100 °C (2,010 °F; 1,370 K) | |||
| Related compounds | |||
| Other cations |
Silicon tetrafluoride Germanium tetrafluoride Tin tetrafluoride Lead tetrafluoride | ||
| Related fluoromethanes |
Fluoromethane Difluoromethane Fluoroform | ||
| Related compounds |
Tetrachloromethane Tetrabromomethane Tetraiodomethane | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Tetrafluoromethane, also known as carbon tetrafluoride, is the simplest fluorocarbon (CF4). It has a very high bond strength due to the nature of the carbon–fluorine bond. It can also be classified as a haloalkane or halomethane. Because of the multiple carbon–fluorine bonds, and the high electronegativity of fluorine, the carbon in tetrafluoromethane has a significant positive partial charge which strengthens and shortens the four carbon–fluorine bonds by providing additional ionic character. Tetrafluoromethane is a potent greenhouse gas.
Bonding
Carbon–fluorine bonds are the strongest single bonds in organic chemistry.[2] Additionally, they strengthen as more carbon–fluorine bonds are added to the same carbon. In the one carbon organofluorine compounds represented by molecules of fluoromethane, difluoromethane, trifluoromethane, and tetrafluoromethane, the carbon–fluorine bonds are strongest in tetrafluoromethane.[3] This effect is due to the increased coulombic attractions between the fluorine atoms and the carbon because the carbon has a positive partial charge of 0.76.[3]
Preparation
Tetrafluoromethane is the product when any carbon compound, including carbon itself, is burned in an atmosphere of fluorine. With hydrocarbons, hydrogen fluoride is a coproduct. It was first reported in 1926.[4] It can also be prepared by the fluorination of carbon dioxide, carbon monoxide or phosgene with sulfur tetrafluoride. Commercially it is manufactured by the reaction of hydrogen fluoride with dichlorodifluoromethane or chlorotrifluoromethane; it is also produced during the electrolysis of metal fluorides MF, MF2 using a carbon electrode.
Although it can be made from myriad precursors and fluorine, elemental fluorine is expensive and difficult to handle. Consequently, CF
4 is prepared on an industrial scale using hydrogen fluoride:[5]
- CCl2F2 + 2 HF → CF4 + 2 HCl
Laboratory synthesis
Tetrafluoromethane can be prepared in the laboratory by the reaction of silicon carbide with fluorine.
- SiC + 4 F2 → CF4 + SiF4
Reactions
Tetrafluoromethane, like other fluorocarbons, is very stable due to the strength of its carbon–fluorine bonds. The bonds in tetrafluoromethane have a bonding energy of 515 kJ⋅mol−1. As a result, it is inert to acids and hydroxides. However, it reacts explosively with alkali metals. Thermal decomposition or combustion of CF4 produces toxic gases (carbonyl fluoride and carbon monoxide) and in the presence of water will also yield hydrogen fluoride.
It is very slightly soluble in water (about 20 mg⋅L−1), but miscible with organic solvents.
Uses
Tetrafluoromethane is sometimes used as a low temperature refrigerant. It is used in electronics microfabrication alone or in combination with oxygen as a plasma etchant for silicon, silicon dioxide, and silicon nitride.[6] It also has uses in neutron detectors [7]
Environmental effects
Tetrafluoromethane is a potent greenhouse gas that contributes to the greenhouse effect. It is very stable, has an atmospheric lifetime of 50,000 years, and a high greenhouse warming potential of 6500 (which is given for the first 100 years thereof, CO2 has a factor of 1); however, the low amount in the atmosphere restricts the overall radiative forcing effect.
Although structurally similar to chlorofluorocarbons (CFCs), tetrafluoromethane does not deplete the ozone layer. This is because the depletion is caused by the chlorine atoms in CFCs, which dissociate when struck by UV radiation. Carbon–fluorine bonds are stronger and less likely to dissociate. According to Guinness World Records Tetrafluoromethane is the most persistent greenhouse gas.
Main industrial emissions of tetrafluoromethane besides hexafluoroethane are produced during production of aluminium using Hall-Héroult process. CF4 also is produced as product of the breakdown of more complex compounds such as halocarbons.[8]
Health risks
Due to its density, tetrafluoromethane can displace air, creating an asphyxiation hazard in inadequately ventilated areas.
See also
References
- ↑ Abjean, R.; A. Bideau-Mehu; Y. Guern (15 July 1990). "Refractive index of carbon tetrafluoride (CF4) in the 300-140 nm wavelength range". Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 292 (3): 593–594. doi:10.1016/0168-9002(90)90178-9.
- ↑ O'Hagan D (February 2008). "Understanding organofluorine chemistry and in cations. An introduction to the C–F bond". Chemical Society Reviews. 37 (2): 308–19. PMID 18197347. doi:10.1039/b711844a.
- 1 2 Lemal, D.M. (2004). "Perspective on Fluorocarbon Chemistry". J. Org. Chem. 69 (1): 1–11. PMID 14703372. doi:10.1021/jo0302556.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0-08-037941-9.
- ↑ G. Siegemund, W. Schwertfeger, A. Feiring, B. Smart, F. Behr, H. Vogel, B. McKusick "Fluorine Compounds, Organic" in "Ullmann’s Encyclopedia of Industrial Chemistry" 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a11_349
- ↑ K. Williams, K. Gupta, M. Wasilik. Etch Rates for Micromachining Processing – Part II J. Microelectromech. Syst., vol. 12, pp. 761–777, December 2003.
- ↑ "Low efficiency 2-dimensional position-sensitive neutron detector for beam profile measurement". Retrieved 19 July 2012.
- ↑ Jubb, Aaron M.; McGillen, Max R.; Portmann, Robert W.; Daniel, John S.; Burkholder, James B. (2015). "An atmospheric photochemical source of the persistent greenhouse gas CF4". Geophysical Research Letters. 42 (21): 9505–9511. ISSN 0094-8276. doi:10.1002/2015GL066193.
External links
- International Chemical Safety Card 0575
- National Pollutant Inventory – Fluoride and compounds fact sheet
- Data from Air Liquide
- Vapor pressure graph at Air Liquide
- MSDS at Oxford University
- Protocol for measurement of tetrafluoromethane and hexafluoroethane from primary aluminium production
- Chemical and physical properties table
- WebBook page for CF4