CA1 (gene)
Carbonic anhydrase 1 is an enzyme that in humans is encoded by the CA1 gene.[3][4]
Carbonic anhydrases (CAs) are a large family of zinc metalloenzymes that catalyze the reversible hydration of carbon dioxide. They participate in a variety of biological processes, including cellular respiration, calcification, acid-base balance, bone resorption, and the formation of aqueous humor, cerebrospinal fluid, saliva, and gastric acid.
They show extensive diversity in tissue distribution and in their subcellular localization. CA1 is closely linked to CA2 and CA3 genes on chromosome 8, and it encodes a cytosolic protein which is found at the highest level in erythrocytes. Transcript variants of CA1 utilizing alternative polyA_sites have been described in literature.[4]
Structure
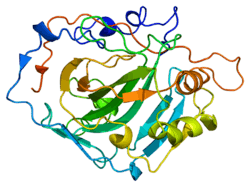
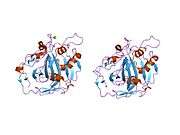
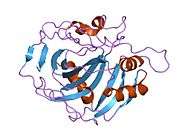
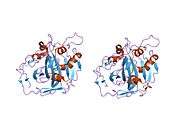
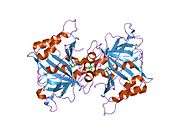
The human CA1 protein contains an N-terminus active site, zinc binding site, and substrate-binding site.[5] The crystal structure of the human CA1-bicarbonate anion complex reveals the geometry of two H-bonds between the Glu106-Thr199 pair and the Glu117-His119 pair, and one pi H-bond between a water molecule and the phenyl ring of the Tyr114 residue. The product inhibition of CA1 via bicarbonate anions is correlated to the proton localization change on His119. So the Glu117-His119 H-bond is considered to regulate the ionicity of the zinc ion and the binding strength of the bicarbonate anion.[6]
Mechanism
The reaction catalyzed by CA1 is the same as other carbonic anhydrase family proteins:
(in tissues - high CO2 concentration)[7]
The CA1-catalyzed reaction has a relatively low reaction affinity (Km) of 4.0 mM for CO2,[5][8] turnover number (Kcat) of 2x10^5 s−1, and catalytic efficiency (Kcat/Km) of 5x10^7 M−1s−1 comparing to other isozymes of the α-CA family of carbonic anhydrases. The turnover rate and catalytic rate of CA1 are only about 10% that of CA2 (Kcat: 1.4x10^6 s−1, Kcat/Km: 1.5x10^8 M−1s−1).[9]
Function
Carbonic anhydrase 1 belongs to α-CA sub-family and is localized in the cytosol of red blood cell, GI tract, cardiac tissues and other organs or tissues.[10] Transmembrane transport of CA-produced bicarbonate contributes significantly to cellular pH regulation.[11]
In a human zinc-activated variant of CA1, the Michigan Variant, a single point mutation changes His 67 to Arg in a critical region of the active site. This variant of the zinc metalloenzyme appears to be unique in that it possesses esterase activity that is specifically enhanced by added free zinc ions.[12]
Clinical significance
CA1 activation is associated with worsened pathological remodeling in human ischemic diabetic cardiomyopathy.[10] In diabetic mellitus type 2 patients with postinfarct heart failure who were undergoing surgical coronary revascularization, myocardial levels of CA1 were sixfold higher than nondiabetic patients. Elevated CA1 expression was mainly localized in the cardiac interstitium and endothelial cells. Furthermore, high glucose-induced elevation of CA1 hampers endothelial cell permeability and determines endothelial cell apoptosis in vitro.[10]
CA1 also mediates hemorrhagic retinal and cerebral vascular permeability through prekallikrein activation and serine protease factor XIIa generation. These phenomena induce proliferative diabetic retinopathy and diabetic macular edema disease progression, which represent leading causes of vision loss.[13]
As CA1 is an important therapeutic target, development of its inhibitors will contribute to disease treatment. Compared to other CA family members, CA1 has relatively low affinity to common CA inhibitors.[14] Nonetheless, it has medium affinity for CA inhibitor sulfonamides.[15]
Interactions
CA1 has been shown to interact with:
These interactions have been confirmed using the high throughput method (one hit)
References
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Lowe N, Edwards YH, Edwards M, Butterworth PH (Aug 1991). "Physical mapping of the human carbonic anhydrase gene cluster on chromosome 8". Genomics. 10 (4): 882–8. PMID 1916821. doi:10.1016/0888-7543(91)90176-F.
- 1 2 "Entrez Gene: CA1 carbonic anhydrase I".
- 1 2 "CA1 - Carbonic anhydrase 1 - Homo sapiens (Human) - CA1 gene & protein". www.uniprot.org. Retrieved 2016-03-23.
- ↑ Kumar V, Kannan KK (Aug 1994). "Enzyme-substrate interactions. Structure of human carbonic anhydrase I complexed with bicarbonate". Journal of Molecular Biology. 241 (2): 226–32. PMID 8057362. doi:10.1006/jmbi.1994.1491.
- ↑ Carbonic acid has a pKa of around 6.36 (the exact value depends on the medium) so at pH 7 a small percentage of the bicarbonate is protonated. See carbonic acid for details concerning the equilibria HCO−
3 + H+ H2CO3 and H2CO3 CO2 + H2O - ↑ Briganti F, Mangani S, Scozzafava A, Vernaglione G, Supuran CT (Oct 1999). "Carbonic anhydrase catalyzes cyanamide hydration to urea: is it mimicking the physiological reaction?". Journal of Biological Inorganic Chemistry. 4 (5): 528–36. PMID 10550681. doi:10.1007/s007750050375.
- ↑ Silverman DN, Lindskog S (2002-05-01). "The catalytic mechanism of carbonic anhydrase: implications of a rate-limiting protolysis of water". Accounts of Chemical Research. 21 (1): 30–36. doi:10.1021/ar00145a005.
- 1 2 3 Torella D, Ellison GM, Torella M, Vicinanza C, Aquila I, Iaconetti C, Scalise M, Marino F, Henning BJ, Lewis FC, Gareri C, Lascar N, Cuda G, Salvatore T, Nappi G, Indolfi C, Torella R, Cozzolino D, Sasso FC (2014-01-01). "Carbonic anhydrase activation is associated with worsened pathological remodeling in human ischemic diabetic cardiomyopathy". Journal of the American Heart Association. 3 (2): e000434. PMC 4187518
 . PMID 24670789. doi:10.1161/JAHA.113.000434.
. PMID 24670789. doi:10.1161/JAHA.113.000434. - ↑ Alvarez BV, Quon AL, Mullen J, Casey JR (2013-01-01). "Quantification of carbonic anhydrase gene expression in ventricle of hypertrophic and failing human heart". BMC Cardiovascular Disorders. 13: 2. PMC 3570296
 . PMID 23297731. doi:10.1186/1471-2261-13-2.
. PMID 23297731. doi:10.1186/1471-2261-13-2. - ↑ Ferraroni M, Tilli S, Briganti F, Chegwidden WR, Supuran CT, Wiebauer KE, Tashian RE, Scozzafava A (May 2002). "Crystal structure of a zinc-activated variant of human carbonic anhydrase I, CA I Michigan 1: evidence for a second zinc binding site involving arginine coordination". Biochemistry. 41 (20): 6237–44. PMID 12009884. doi:10.1021/bi0120446.
- ↑ Gao BB, Clermont A, Rook S, Fonda SJ, Srinivasan VJ, Wojtkowski M, Fujimoto JG, Avery RL, Arrigg PG, Bursell SE, Aiello LP, Feener EP (Feb 2007). "Extracellular carbonic anhydrase mediates hemorrhagic retinal and cerebral vascular permeability through prekallikrein activation". Nature Medicine. 13 (2): 181–8. PMID 17259996. doi:10.1038/nm1534.
- ↑ Supuran CT (Feb 2008). "Carbonic anhydrases: novel therapeutic applications for inhibitors and activators". Nature Reviews. Drug Discovery. 7 (2): 168–81. PMID 18167490. doi:10.1038/nrd2467.
- ↑ Şentürk M. "Carbonic Anhydrase Inhibitors and Activators: Small Organic Molecules as Drugs and Prodrugs" (PDF).
- ↑ Rolland T, Taşan M, Charloteaux B, Pevzner SJ, Zhong Q, Sahni N, et al. (Nov 2014). "A proteome-scale map of the human interactome network". Cell. 159 (5): 1212–26. PMC 4266588
 . PMID 25416956. doi:10.1016/j.cell.2014.10.050.
. PMID 25416956. doi:10.1016/j.cell.2014.10.050. - ↑ Wang J, Huo K, Ma L, Tang L, Li D, Huang X, et al. (2011-01-01). "Toward an understanding of the protein interaction network of the human liver". Molecular Systems Biology. 7: 536. PMC 3261708
 . PMID 21988832. doi:10.1038/msb.2011.67.
. PMID 21988832. doi:10.1038/msb.2011.67. - ↑ Vinayagam A, Stelzl U, Foulle R, Plassmann S, Zenkner M, Timm J, Assmus HE, Andrade-Navarro MA, Wanker EE (Sep 2011). "A directed protein interaction network for investigating intracellular signal transduction". Science Signaling. 4 (189): rs8. PMID 21900206. doi:10.1126/scisignal.2001699.
External links
- Human CA1 genome location and CA1 gene details page in the UCSC Genome Browser.
Further reading
- Tashian RE, Carter ND (1977). "Biochemical genetics of carbonic anhydrase". Advances in Human Genetics. 7: 1–56. PMID 827930.
- Sly WS, Hu PY (1995). "Human carbonic anhydrases and carbonic anhydrase deficiencies". Annual Review of Biochemistry. 64 (1): 375–401. PMID 7574487. doi:10.1146/annurev.bi.64.070195.002111.
- Kendall AG, Tashian RE (Jul 1977). "Erythrocyte carbonic anhydrase I: inherited deficiency in humans". Science. 197 (4302): 471–2. PMID 406674. doi:10.1126/science.406674.
- Kannan KK, Notstrand B, Fridborg K, Lövgren S, Ohlsson A, Petef M (Jan 1975). "Crystal structure of human erythrocyte carbonic anhydrase B. Three-dimensional structure at a nominal 2.2-A resolution". Proceedings of the National Academy of Sciences of the United States of America. 72 (1): 51–5. PMC 432238
 . PMID 804171. doi:10.1073/pnas.72.1.51.
. PMID 804171. doi:10.1073/pnas.72.1.51. - Dawson SJ, White LA (May 1992). "Treatment of Haemophilus aphrophilus endocarditis with ciprofloxacin". The Journal of Infection. 24 (3): 317–20. PMID 1602151. doi:10.1016/S0163-4453(05)80037-4.
- Lowe N, Brady HJ, Barlow JH, Sowden JC, Edwards M, Butterworth PH (Sep 1990). "Structure and methylation patterns of the gene encoding human carbonic anhydrase I". Gene. 93 (2): 277–83. PMID 2121614. doi:10.1016/0378-1119(90)90236-K.
- Noda Y, Sumitomo S, Hikosaka N, Mori M (Apr 1986). "Immunohistochemical observations on carbonic anhydrase I and II in human salivary glands and submandibular obstructive adenitis". Journal of Oral Pathology. 15 (4): 187–90. PMID 3088232. doi:10.1111/j.1600-0714.1986.tb00604.x.
- Barlow JH, Lowe N, Edwards YH, Butterworth PH (Mar 1987). "Human carbonic anhydrase I cDNA". Nucleic Acids Research. 15 (5): 2386. PMC 340641
 . PMID 3104879. doi:10.1093/nar/15.5.2386.
. PMID 3104879. doi:10.1093/nar/15.5.2386. - Edwards YH, Barlow JH, Konialis CP, Povey S, Butterworth PH (May 1986). "Assignment of the gene determining human carbonic anhydrase, CAI, to chromosome 8". Annals of Human Genetics. 50 (Pt 2): 123–9. PMID 3124707. doi:10.1111/j.1469-1809.1986.tb01030.x.
- Lin KT, Deutsch HF (Apr 1974). "Human carbonic anhydrases. XII. The complete primary structure of the C isozyme". The Journal of Biological Chemistry. 249 (8): 2329–37. PMID 4207120.
- Giraud N, Marriq C, Laurent-Tabusse G (1975). "[Primary structure of human B erythrocyte carbonic anhydrase. 3. Sequence of CNBr fragment I and III (residues 149-260)]". Biochimie. 56 (8): 1031–43. PMID 4217196. doi:10.1016/S0300-9084(74)80093-3.
- Andersson B, Nyman PO, Strid L (Aug 1972). "Amino acid sequence of human erythrocyte carbonic anhydrase B". Biochemical and Biophysical Research Communications. 48 (3): 670–7. PMID 4625868. doi:10.1016/0006-291X(72)90400-7.
- Lin KT, Deutsch HF (Mar 1973). "Human carbonic anhydrases. XI. The complete primary structure of carbonic anhydrase B". The Journal of Biological Chemistry. 248 (6): 1885–93. PMID 4632246.
- Omoto K, Ueda S, Goriki K, Takahashi N, Misawa S, Pagaran IG (Jan 1981). "Population genetic studies of the Philippine Negritos. III. Identification of the carbonic anhydrase-1 variant with CA1 Guam". American Journal of Human Genetics. 33 (1): 105–11. PMC 1684865
 . PMID 6781336.
. PMID 6781336. - Chegwidden WR, Wagner LE, Venta PJ, Bergenhem NC, Yu YS, Tashian RE (1995). "Marked zinc activation of ester hydrolysis by a mutation, 67-His (CAT) to Arg (CGT), in the active site of human carbonic anhydrase I". Human Mutation. 4 (4): 294–6. PMID 7866410. doi:10.1002/humu.1380040411.
- Bekku S, Mochizuki H, Takayama E, Shinomiya N, Fukamachi H, Ichinose M, Tadakuma T, Yamamoto T (Dec 1998). "Carbonic anhydrase I and II as a differentiation marker of human and rat colonic enterocytes". Research in Experimental Medicine. Zeitschrift Für Die Gesamte Experimentelle Medizin Einschliesslich Experimenteller Chirurgie. 198 (4): 175–85. PMID 9879596.
- Puscas I, Coltau M, Baican M, Pasca R, Domuta G, Hecht A (2001). "Vasoconstrictive drugs increase carbonic anhydrase I in vascular smooth muscle while vasodilating drugs reduce the activity of this isozyme by a direct mechanism of action". Drugs Under Experimental and Clinical Research. 27 (2): 53–60. PMID 11392054.