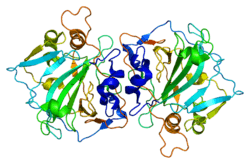
CA12
| CA12 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||
| |||||||||||||||||
| Identifiers | |||||||||||||||||
| Aliases | CA12, CAXII, HsT18816, CA-XII, T18816, carbonic anhydrase 12 | ||||||||||||||||
| External IDs | MGI: 1923709 HomoloGene: 20327 GeneCards: CA12 | ||||||||||||||||
| |||||||||||||||||
| RNA expression pattern | |||||||||||||||||
   | |||||||||||||||||
| More reference expression data | |||||||||||||||||
| Orthologs | |||||||||||||||||
| Species | Human | Mouse | |||||||||||||||
| Entrez | |||||||||||||||||
| Ensembl | |||||||||||||||||
| UniProt | |||||||||||||||||
| RefSeq (mRNA) | |||||||||||||||||
| RefSeq (protein) | |||||||||||||||||
| Location (UCSC) | Chr 15: 63.32 – 63.38 Mb | Chr 9: 66.71 – 66.77 Mb | |||||||||||||||
| PubMed search | [1] | [2] | |||||||||||||||
| Wikidata | |||||||||||||||||
| |||||||||||||||||
Carbonic anhydrase 12 is an enzyme that in humans is encoded by the CA12 gene.[3][4]
Function
Carbonic anhydrases (CAs) are a large family of zinc metalloenzymes that catalyze the reversible hydration of carbon dioxide. They participate in a variety of biological processes, including respiration, calcification, acid-base balance, bone resorption, and the formation of aqueous humor, cerebrospinal fluid, saliva, and gastric acid. This gene product is a type I membrane protein that is highly expressed in normal tissues, such as kidney, colon and pancreas, and has been found to be overexpressed in 10% of clear cell renal carcinomas. Two transcript variants encoding different isoforms have been identified for this gene.[4]
Pathology
Loss of function mutations in the CAXII gene result in defects in fluids and carbonate secretions in the following diseases:
1) Cystic fibrosis-like syndrome with normal cystic fibrosis transmembrane conductance regulator (CFTR) protein levels [5][6][7][8][9][10]
4) Xerostomia or dry mouth syndrome[7][8][9]
Molecular Basis of Cystic Fibrosis-like Syndrome
CAXII, with either the His121Gln or Glu143Lys mutation, localizes to basolateral membranes of polarized MDCK cells similar to the wild type enzyme, indicating no deleterious effect on subcellular location.[12]
However, CAXII mutant enzymes show reduced activity. These observations made it very hard to explain the mechanism for the autosomal recessive disorder of hyponatremia, causing salt wasting in sweat due to mutant CAXII.[5][6]
In a separate study, researchers observed that mutant enzyme activity is completely reduced at physiological concentrations of sodium chloride.[12] Thus, loss of the function of CAXII in sweat glands and lungs is the molecular basis for cystic fibrosis patients with normal CFTR levels.[12]
High Impact Information on CAXII
Differential modulation of the active site environment of CAXII by cationic quantum dots and polylysine helps design CAXII specific activators and inhibitors of the enzyme.[14] CAXII specific inhibition provides a tool to interfere with cell proliferation, resulting in cell apoptosis in T-cell lymphomas.[15]
Analytical, Diagnostic, and Therapeutic Context of CAXII
Serum CAXII levels should be applicable as a sero-diagnostic marker for lung cancer.[16]
References
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Türeci O, Sahin U, Vollmar E, Siemer S, Göttert E, Seitz G, Parkkila AK, Shah GN, Grubb JH, Pfreundschuh M, Sly WS (Aug 1998). "Human carbonic anhydrase XII: cDNA cloning, expression, and chromosomal localization of a carbonic anhydrase gene that is overexpressed in some renal cell cancers". Proc Natl Acad Sci U S A. 95 (13): 7608–7613. PMC 22698
 . PMID 9636197. doi:10.1073/pnas.95.13.7608.
. PMID 9636197. doi:10.1073/pnas.95.13.7608. - 1 2 "Entrez Gene: CA12 carbonic anhydrase XII".
- 1 2 Feldshtein, M; Elkrinawi, S; Yerushalmi, B; Marcus, B; Vullo, D; Romi, H; Ofir, R; Landau, D; Sivan, S; Supuran, CT; Birk, OS (12 November 2010). "Hyperchlorhidrosis caused by homozygous mutation in CA12, encoding carbonic anhydrase XII.". American Journal of Human Genetics. 87 (5): 713–20. PMC 2978943
 . PMID 21035102. doi:10.1016/j.ajhg.2010.10.008.
. PMID 21035102. doi:10.1016/j.ajhg.2010.10.008. - 1 2 Muhammad, E; Leventhal, N; Parvari, G; Hanukoglu, A; Hanukoglu, I; Chalifa-Caspi, V; Feinstein, Y; Weinbrand, J; Jacoby, H; Manor, E; Nagar, T; Beck, JC; Sheffield, VC; Hershkovitz, E; Parvari, R (April 2011). "Autosomal recessive hyponatremia due to isolated salt wasting in sweat associated with a mutation in the active site of Carbonic Anhydrase 12.". Human Genetics. 129 (4): 397–405. PMID 21184099. doi:10.1007/s00439-010-0930-4.
- 1 2 Hong, JH; Muhammad, E; Zheng, C; Hershkovitz, E; Alkrinawi, S; Loewenthal, N; Parvari, R; Muallem, S (15 December 2015). "Essential role of carbonic anhydrase XII in secretory gland fluid and HCO3 (-) secretion revealed by disease causing human mutation.". The Journal of Physiology. 593 (24): 5299–312. PMID 26486891. doi:10.1113/jp271378.
- 1 2 Lee, M; Vecchio-Pagán, B; Sharma, N; Waheed, A; Li, X; Raraigh, KS; Robbins, S; Han, ST; Franca, AL; Pellicore, MJ; Evans, TA; Arcara, KM; Nguyen, H; Luan, S; Belchis, D; Hertecant, J; Zabner, J; Sly, WS; Cutting, GR (23 February 2016). "Loss of carbonic anhydrase XII function in individuals with elevated sweat chloride concentration and pulmonary airway disease.". Human Molecular Genetics. 25: 1923–1933. PMID 26911677. doi:10.1093/hmg/ddw065.
- 1 2 Purkerson, JM; Schwartz, GJ (January 2007). "The role of carbonic anhydrases in renal physiology.". Kidney International. 71 (2): 103–15. PMID 17164835. doi:10.1038/sj.ki.5002020.
- ↑ Quinton, PM (December 2010). "Role of epithelial HCO3⁻ transport in mucin secretion: lessons from cystic fibrosis.". American Journal of Physiology. Cell Physiology. 299 (6): C1222–33. PMC 3006319
 . PMID 20926781. doi:10.1152/ajpcell.00362.2010.
. PMID 20926781. doi:10.1152/ajpcell.00362.2010. - ↑ Kivelä, AJ; Parkkila, S; Saarnio, J; Karttunen, TJ; Kivelä, J; Parkkila, AK; Pastoreková, S; Pastorek, J; Waheed, A; Sly, WS; Rajaniemi, H (September 2000). "Expression of transmembrane carbonic anhydrase isoenzymes IX and XII in normal human pancreas and pancreatic tumours.". Histochemistry and cell biology. 114 (3): 197–204. PMID 11083462. doi:10.1007/s004180000181.
- 1 2 3 4 5 Lee, MG; Ohana, E; Park, HW; Yang, D; Muallem, S (January 2012). "Molecular mechanism of pancreatic and salivary gland fluid and HCO3 secretion.". Physiological reviews. 92 (1): 39–74. PMC 3667394
 . PMID 22298651. doi:10.1152/physrev.00011.2011.
. PMID 22298651. doi:10.1152/physrev.00011.2011. - ↑ Almståhl, A; Wikström, M (May 2003). "Electrolytes in stimulated whole saliva in individuals with hyposalivation of different origins.". Archives of oral biology. 48 (5): 337–44. PMID 12711377. doi:10.1016/s0003-9969(02)00200-5.
- ↑ Manokaran, S; Zhang, X; Chen, W; Srivastava, DK (June 2010). "Differential modulation of the active site environment of human carbonic anhydrase XII by cationic quantum dots and polylysine.". Biochimica et Biophysica Acta. 1804 (6): 1376–84. PMC 3181075
 . PMID 20215053. doi:10.1016/j.bbapap.2010.02.014.
. PMID 20215053. doi:10.1016/j.bbapap.2010.02.014. - ↑ Lounnas, N; Rosilio, C; Nebout, M; Mary, D; Griessinger, E; Neffati, Z; Chiche, J; Spits, H; Hagenbeek, TJ; Asnafi, V; Poulsen, SA; Supuran, CT; Peyron, JF; Imbert, V (1 June 2013). "Pharmacological inhibition of carbonic anhydrase XII interferes with cell proliferation and induces cell apoptosis in T-cell lymphomas.". Cancer Letters. 333 (1): 76–88. PMID 23348702. doi:10.1016/j.canlet.2013.01.020.
- ↑ Kobayashi, M; Matsumoto, T; Ryuge, S; Yanagita, K; Nagashio, R; Kawakami, Y; Goshima, N; Jiang, SX; Saegusa, M; Iyoda, A; Satoh, Y; Masuda, N; Sato, Y (2012). "CAXII Is a sero-diagnostic marker for lung cancer.". PLOS ONE. 7 (3): e33952. PMC 3306309
 . PMID 22439015. doi:10.1371/journal.pone.0033952.
. PMID 22439015. doi:10.1371/journal.pone.0033952.
External links
- Human CA12 genome location and CA12 gene details page in the UCSC Genome Browser.
Further reading
- Sahin U, Türeci O, Schmitt H, Cochlovius B, Johannes T, Schmits R, Stenner F, Luo G, Schobert I, Pfreundschuh M (1996). "Human neoplasms elicit multiple specific immune responses in the autologous host". Proc. Natl. Acad. Sci. U.S.A. 92 (25): 11810–11813. PMC 40492
 . PMID 8524854. doi:10.1073/pnas.92.25.11810.
. PMID 8524854. doi:10.1073/pnas.92.25.11810. - Ivanov SV, Kuzmin I, Wei MH, Pack S, Geil L, Johnson BE, Stanbridge EJ, Lerman MI (1998). "Down-regulation of transmembrane carbonic anhydrases in renal cell carcinoma cell lines by wild-type von Hippel-Lindau transgenes". Proc. Natl. Acad. Sci. U.S.A. 95 (21): 12596–12601. PMC 22876
 . PMID 9770531. doi:10.1073/pnas.95.21.12596.
. PMID 9770531. doi:10.1073/pnas.95.21.12596. - Fujikawa-Adachi K, Nishimori I, Taguchi T, Onishi S (1999). "Human carbonic anhydrase XIV (CA14): cDNA cloning, mRNA expression, and mapping to chromosome 1". Genomics. 61 (1): 74–81. PMID 10512682. doi:10.1006/geno.1999.5938.
- Karhumaa P, Parkkila S, Türeci O, Waheed A, Grubb JH, Shah G, Parkkila A, Kaunisto K, Tapanainen J, Sly WS, Rajaniemi H (2000). "Identification of carbonic anhydrase XII as the membrane isozyme expressed in the normal human endometrial epithelium". Mol. Hum. Reprod. 6 (1): 68–74. PMID 10611263. doi:10.1093/molehr/6.1.68.
- Kivelä A, Parkkila S, Saarnio J, Karttunen TJ, Kivelä J, Parkkila AK, Waheed A, Sly WS, Grubb JH, Shah G, Türeci O, Rajaniemi H (2000). "Expression of a novel transmembrane carbonic anhydrase isozyme XII in normal human gut and colorectal tumors". Am. J. Pathol. 156 (2): 577–84. PMC 1850052
 . PMID 10666387. doi:10.1016/S0002-9440(10)64762-1.
. PMID 10666387. doi:10.1016/S0002-9440(10)64762-1. - Kivelä AJ, Parkkila S, Saarnio J, Karttunen TJ, Kivelä J, Parkkila AK, Pastoreková S, Pastorek J, Waheed A, Sly WS, Rajaniemi H (2001). "Expression of transmembrane carbonic anhydrase isoenzymes IX and XII in normal human pancreas and pancreatic tumours". Histochem. Cell Biol. 114 (3): 197–204. PMID 11083462. doi:10.1007/s004180000181.
- Parkkila S, Parkkila AK, Saarnio J, Kivelä J, Karttunen TJ, Kaunisto K, Waheed A, Sly WS, Türeci O, Virtanen I, Rajaniemi H (2001). "Expression of the membrane-associated carbonic anhydrase isozyme XII in the human kidney and renal tumors". J. Histochem. Cytochem. 48 (12): 1601–8. PMID 11101628. doi:10.1177/002215540004801203.
- Wykoff CC, Beasley N, Watson PH, Campo L, Chia SK, English R, Pastorek J, Sly WS, Ratcliffe P, Harris AL (2001). "Expression of the hypoxia-inducible and tumor-associated carbonic anhydrases in ductal carcinoma in situ of the breast". Am. J. Pathol. 158 (3): 1011–9. PMC 1850356
 . PMID 11238049. doi:10.1016/S0002-9440(10)64048-5.
. PMID 11238049. doi:10.1016/S0002-9440(10)64048-5. - Karhumaa P, Kaunisto K, Parkkila S, Waheed A, Pastoreková S, Pastorek J, Sly WS, Rajaniemi H (2001). "Expression of the transmembrane carbonic anhydrases, CA IX and CA XII, in the human male excurrent ducts". Mol. Hum. Reprod. 7 (7): 611–616. PMID 11420383. doi:10.1093/molehr/7.7.611.
- Whittington DA, Waheed A, Ulmasov B, Shah GN, Grubb JH, Sly WS, Christianson DW (2001). "Crystal structure of the dimeric extracellular domain of human carbonic anhydrase XII, a bitopic membrane protein overexpressed in certain cancer tumor cells". Proc. Natl. Acad. Sci. U.S.A. 98 (17): 9545–9550. PMC 55489
 . PMID 11493685. doi:10.1073/pnas.161301298.
. PMID 11493685. doi:10.1073/pnas.161301298. - Kivela AJ, Saarnio J, Karttunen TJ, Kivelä J, Parkkila AK, Pastorekova S, Pastorek J, Waheed A, Sly WS, Parkkila TS, Rajaniemi H (2001). "Differential expression of cytoplasmic carbonic anhydrases, CA I and II, and membrane-associated isozymes, CA IX and XII, in normal mucosa of large intestine and in colorectal tumors". Dig. Dis. Sci. 46 (10): 2179–2186. PMID 11680594. doi:10.1023/A:1011910931210.
- Liao SY, Ivanov S, Ivanova A, Ghosh S, Cote MA, Keefe K, Coca-Prados M, Stanbridge EJ, Lerman MI (2003). "Expression of cell surface transmembrane carbonic anhydrase genes CA9 and CA12 in the human eye: overexpression of CA12 (CAXII) in glaucoma". J. Med. Genet. 40 (4): 257–261. PMC 1735430
 . PMID 12676895. doi:10.1136/jmg.40.4.257.
. PMID 12676895. doi:10.1136/jmg.40.4.257. - Leppilampi M, Saarnio J, Karttunen TJ, Kivelä J, Pastoreková S, Pastorek J, Waheed A, Sly WS, Parkkila S (2003). "Carbonic anhydrase isozymes IX and XII in gastric tumors". World J. Gastroenterol. 9 (7): 1398–403. PMID 12854129.
- Kyllönen MS, Parkkila S, Rajaniemi H, Waheed A, Grubb JH, Shah GN, Sly WS, Kaunisto K (2003). "Localization of carbonic anhydrase XII to the basolateral membrane of H+-secreting cells of mouse and rat kidney". J. Histochem. Cytochem. 51 (9): 1217–24. PMID 12923247. doi:10.1177/002215540305100912.
- Tarun AS, Bryant B, Zhai W, Solomon C, Shusterman D (2004). "Gene expression for carbonic anhydrase isoenzymes in human nasal mucosa". Chem. Senses. 28 (7): 621–629. PMID 14578124. doi:10.1093/chemse/bjg054.
- Kivela AJ, Parkkila S, Saarnio J, Karttunen TJ, Kivela J, Parkkila AK, Bartosova M, Mucha V, Novak M, Waheed A, Sly WS, Rajaniemi H, Pastorekova S, Pastorek J (2005). "Expression of von Hippel-Lindau tumor suppressor and tumor-associated carbonic anhydrases IX and XII in normal and neoplastic colorectal mucosa". World J. Gastroenterol. 11 (17): 2616–25. PMID 15849821.

