Carbon–hydrogen bond activation

Carbon–hydrogen bond functionalization (C–H functionalization) is a type of reaction in which a carbon–hydrogen bond is cleaved and replaced with a carbon-X bond (where X is usually carbon, oxygen, or nitrogen). The term usually implies that a transition metal is involved in the C-H cleavage process.[1][2] Reactions classified by the term typically involve the hydrocarbon first to react with a metal catalyst to create an organometallic complex in which the hydrocarbon is coordinated to the inner-sphere of a metal, either via an intermediate "alkane or arene complex" or as a transition state leading to a "M−C" intermediate.[3][4][5] The intermediate of this first step (known as C-H activation and sometimes used interchangeably with C-H functionalization) can then undergo subsequent reactions to produce the functionalized product. Important to this definition is the requirement that during the C–H cleavage event, the hydrocarbyl species remains associated in the inner-sphere and under the influence of "M".
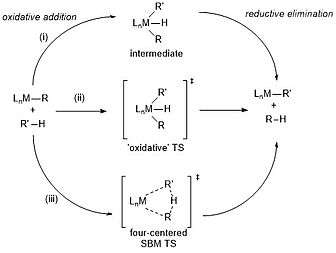
Mechanisms for C-H activations fall under three general categories: (i) oxidative addition, in which a metal center inserts into a carbon-hydrogen bond, which cleaves the bond and oxidizes the metal, producing an intermediate that can undergo reductive elimination to yield the organometallic reactive intermediate (ii) electrophilic activation, which reacts similarly to oxidative addition, but differs in that it produces the organometallic reactive intermediate through an "oxidative" transition state instead of an intermediate, and (iii) Sigma-bond metathesis, which proceeds through a "four-centered" transition state in which bonds break and form in a single step: the target hydrocarbon bond breaks as the carbon bonds to the metal and the hydrogen bonds to one of the metal’s ligands, which causes bond breakage between the ligand and the metal.
C–H bonds, which are traditionally considered unreactive, can be cleaved by coordination. Much research has been devoted to the design and synthesis of new reagents and catalysts that can effect C–H activation. C-H activation chemistry has the potential to transform the chemical world through the development of novel synthetic methods. C-H activation could enable the conversion of cheap and abundant alkanes into valuable functionalized organic compounds and the efficient structural editing of already complex molecules (i.e. natural product synthesis).[6] Selective activation of a specific C-H bond poses a great challenge. In addition to a high bond dissociation energy, C-H bonds have very low polarity because to these two elements have similar electronegativities.
Historic overview
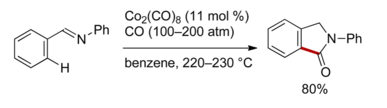
The first C–H activation reaction is often attributed to Otto Dimroth, who in 1902, reported that benzene reacted with mercury(II) acetate (See: organomercury), but some scholars do not view this reaction as being true C–H activation. Many electrophilic metal centers undergo this reaction. Joseph Chatt has been credited by many to be the first to perform the first C-H activation reaction in 1965[7] with the insertion of ruthenium, in the form of RuCl2(dmpe)2 (where dmpe = 1,2-Bis(dimethylphosphino)ethane), into the C-H bond of naphthalene. However, in 1955,[8] Shunsuke Murahashi reported a cobalt-catalyzed chelation-assisted C-H functionalization of 2-phenylisoindolin-1-one from (E)-N,1-diphenylmethanimine.
In 1969, A.E. Shilov reported that potassium tetrachloroplatinate induced isotope scrambling between methane and heavy water. The pathway was proposed to involve binding of methane to Pt(II). In 1972, the Shilov group was able to produce methanol and methyl chloride in a similar reaction involving a stoichiometric amount of potassium tetrachloroplatinate, catalytic potassium hexachloroplatinate, methane and water. Due to the fact that Shilov worked and published in the Soviet Union during the Cold War era, his work was largely ignored by Western scientists. This so-called Shilov system is today one of the few true catalytic systems for alkane functionalizations.[3][9]
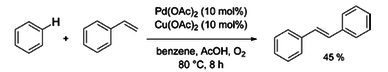
In some cases, discoveries in C-H activation were being made in conjunction with those of cross coupling. In 1969,[10] Yuzo Fujiwara reported the synthesis of (E)-1,2-diphenylethene from benzene and styrene with Pd(OAc)2 and Cu(OAc)2, a procedure very similar to that of cross coupling. On the category of oxidative addition, M. L. H. Green in 1970 reported on the photochemical insertion of tungsten (as a Cp2WH2 complex) in a benzene C–H bond[11] and George M. Whitesides in 1979 was the first to carry out an intramolecular aliphatic C–H activation[12]
The next breakthrough was reported independently by two research groups in 1982. R. G. Bergman reported the first transition metal-mediated intermolecular C–H activation of unactivated and completely saturated hydrocarbons by oxidative addition. Using a photochemical approach, photolysis of Cp*Ir(PMe3)H2, where Cp* is a pentamethylcyclopentadienyl ligand, led to the coordinatively unsaturated species Cp*Ir(PMe3) which reacted via oxidative addition with cyclohexane and neopentane to form the corresponding hydridoalkyl complexes, Cp*Ir(PMe3)HR, where R = cyclohexyl and neopentyl, respectively.[13] W.A.G. Graham found that the same hydrocarbons react with Cp*Ir(CO)2 upon irradiation to afford the related alkylhydrido complexes Cp*Ir(CO)HR, where R = cyclohexyl and neopentyl, respectively.[14] In the latter example, the reaction is presumed to proceed via the oxidative addition of alkane to a 16-electron iridium(I) intermediate, Cp*Ir(CO), formed by irradiation of Cp*Ir(CO)2.
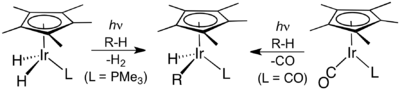 C–H activation by Bergman et al. (left) and Graham et al.
C–H activation by Bergman et al. (left) and Graham et al.
The selective activation and functionalization of alkane C–H bonds was reported using a tungsten complex outfitted with pentamethylcyclopentadienyl, nitrosyl, allyl and neopentyl ligands, Cp*W(NO)(η3-allyl)(CH2CMe3).[15]
In one example involving this system, the alkane pentane is selectively converted to the halocarbon 1-iodopentane. This transformation was achieved via the thermoloysis of Cp*W(NO)(η3-allyl)(CH2CMe3) in pentane at room temperature, resulting in elimination of neopentane by a pseudo-first-order process, generating an undetectable electronically and sterically unsaturated 16-electron intermediate that is coordinated by an η2-butadiene ligand. Subsequent intermolecular activation of a pentane solvent molecule then yields an 18-electron complex possessing an n-pentyl ligand. In a separate step, reaction with iodine at −60 °C liberates 1-iodopentane from the complex.
Arene C–H bonds are readily activated by metal complexes, as illustrated by directed ortho metalation. N,N-dimethylbenzylamine in a cyclometalation readily by many transition metals:[16]
- One manifestation is the Murai olefin coupling.[17]

Scope
Overview
Two pathways achieve C-H activation: innate C-H activation or guided C-H activation. The figure depicts the two oxidation products possible, depending on the reagents used to functionalize a specific C-H bond.[18]
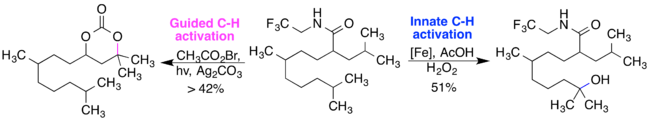
Selectivity of C-H activation
Among the various factors that affect selectivity in C-H activation, the more sterically accessible C-H bond will have more selectivity at that position.

In the figure above, the axial C-H bond in cis-1,2-dimethylcyclohexane is selectively oxidized over the equatorial C-H bond. During oxidation, the axial methyl group becomes planar, decreasing the 1,3-diaxial interactions in the transition state, which stabilizes the transition state.[19]
An alkene C–H bond activation with a rhodium catalyst is demonstrated in the synthesis of this strained bicyclic enamine:[20]
Innate selectivity
Innate selectivity is observed for reactions that functionalize C-H bonds that lack an influence of directing forces, thus only relying on the natural reactivity of the molecule.[18] Inductive (through-bond) effects can explain the innate selectivity of a C-H bond in a molecule through the examination of the electronic nature of the bonds. The presence and proximity of electron-withdrawing groups (EWGs) or electron-donating groups (EDGs) can heavily influence the electron density of a C-H bond.[21] The reactivity trend for nonmetal insertion is tertiary>secondary>primary.[22] Steric effects can also influence the selectivity of C-H bond activation: bulky groups can decrease the rate of functionalizing an adjacent C-H bond.

For oxaziridine (left), the 1st site is oxidized preferably due not only to distance from the electron-withdrawing OBz group but also because of its substitution—the carbon is tertiary—and so it is more electron rich. Similarly, the secondary methylene position in trifluoromethyldioxirane (TFDO, right) that is farthest away to the electron-withdrawing group is selectively oxidized.[21] Note that here, like with oxaziridine, substitution at the carbon also greatly affects selectivity; the secondary methylene position is preferred over the terminal methylene position because the former has a secondary carbon while the latter has a primary one.

In the oxidation of cyclohexane compound, the tertiary site on the ring is preferential over the tertiary site of the isopropyl substituent. The two methyl groups hinder the oxidation on the isopropyl group, making the less hindered cyclohexyl site more favorable.[21]
Guided selectivity
In contrast to innate selectivity, guided selectivity results from external reagents or directing groups affecting the nature of specific C-H bonds.[18]

The figure above demonstrates the use of a pyridine directing group to activate selectively a C-H bond to form a C-halogen bond. Pyridine derivatives are commonly used for ortho-selective C-H functionalization. Such reactions use metals like palladium to catalyze sp2 C-H activation. A similar system uses pyridine to acetoxylate the C-H bond, forming a C-OAc bond, instead of a C-X halogen bond.[2]
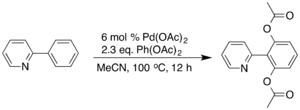
The mechanism for the pyridine based Pd-catalyzed C-H activation reactions involves a catalytic cycle in which the ligand directs the molecule to interact with Pd to form a metallacycle intermediate. The intermediate is oxidized to form a PdIV species, followed by reductive elimination to form the C-O bond and release the product.[2]
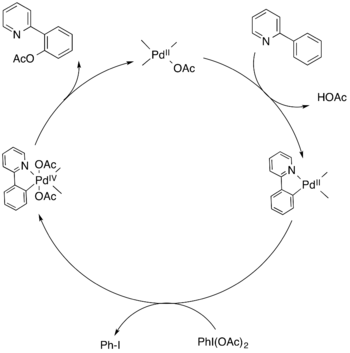
See Meta-selective C-H functionalization for more examples of directed C-H activation.
Reaction conditions
Many C–H bond activations proceed under rather harsh reaction conditions (high temperature, strongly acidic or basic conditions, strong oxidant, etc.), significantly limiting their utility. However, mild methods have been developed, significantly expanding the scope of these transformations.[23] Organocatalysis is another important approach to facilitating C-H activation.[24]
Case Study: Borylation
Transforming C-H bonds into C-B bonds through borylation has been thoroughly investigated due to their utility in synthesis (i.e. for cross-coupling reactions). J.F. Hartwig reported a highly regioselective arene and alkane borylation catalyzed by a rhodium complex. In the case of alkanes, exclusive terminal functionalization was observed.[25]
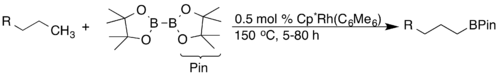
Later, ruthenium catalysts were discovered to have higher activity and functional group compatibility.[26]

Other borylation catalysts have also been developed, including iridium-based catalysts, which successfully activate C-H bonds with high compatibility.[27][28][29]
For more information, consult borylation.
Applications
No applications have been commercialized for homogeneously catalyzed C-H activations.
Natural gas
Naturally occurring methane is not utilized as a chemical feedstock, despite its abundance and low cost. Current technology makes prodigious use of methane by steam reforming to produce syngas, a mixture of carbon monoxide and hydrogen. This syngas is then used in Fischer-Tropsch reactions to make longer carbon chain products or methanol, one of the most important industrial chemical feedstocks.[30][31] An intriguing methods to convert these hydrocarbons involves C-H activation. Periana, for example, reported that complexes containing late transition metals, such as Pt, Pd, Au, and Hg, react with methane (CH4) in H2SO4 to yield methyl bisulfate.[32][33] The process has not however been implemented commercially.
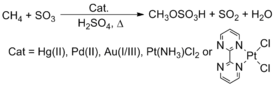
Natural product synthesis
The development of methodology for C-H activation has significantly impacted natural product synthesis, at least in the academic realm. Ideally, synthetic routes contain minimal steps, while maximizing yield. C-H activation has enabled researchers to activate C-H bonds in highly functionalized molecules.[34]
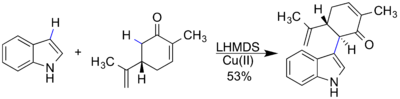
The product in the above reaction is a common scaffold for multiple natural products, including hapalindole Q and ambiguine H. The core structure can be made through a C-C bond (blue bond in product) formation via C-H activation. The innate reactivity of the indole and enolate leads to the formation of the C-C bond to form the indole-carvone intermediate.[18]
(+)-Lithospermic acid via C-H activation
The total synthesis of lithospermic acid employs guided C-H functionalization late stage to a highly functionalized system. The directing group, a chiral nonracemic imine, is capable of performing an intramolecular alkylation, which allows for the rhodium-catalyzed conversion of imine to the dihydrobenzofuran.[35]

Calothrixin A and B via C-H activation
The total synthesis of calothrixin A and B features an intramolecular Pd-catalyzed cross coupling reaction via C-H activation, an example of a guided C-H activation. Cross coupling occurs between aryl C-I and C-H bonds to form a C-C bond (highlighted in red).[36]

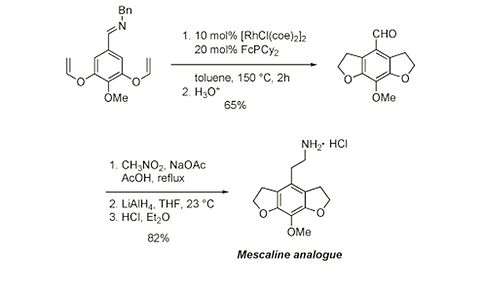
Mescaline analogue via C-H activation
The synthesis of a Mescaline analogue, which has interesting biological properties, employs, specifically, the rhodium-catalyzed enantioselective annulation of an aryl imine via a C-H activation.[37]
See also
Additional Sources
- Bergman FAQ in Nature on C-H activation (2007)
- Literature Presentation by Ramtohul in Stoltz group on applications of C-H activation
- Powerpoint on John Bercaw's work
- Center for Selective C-H Functionalization
References
- ↑ Crabtree, R. H. (2001). "Alkane C–H activation and functionalization with homogeneous transition metal catalysts: a century of progress – a new millennium in prospect". J. Chem. Soc., Dalton Trans. 17: 2437–2450. doi:10.1039/B103147N., Hashiguchi, B. G.; Bischof, S. M.; Konnick, M. M.; Periana, R. A. (2012). "Designing Catalysts for Functionalization of Unactivated C–H Bonds Based on the CH Activation Reaction". Acc. Chem. Res. 45: 885–898. doi:10.1021/ar200250r., Crabtree, R. H. (2004). "Organometallic alkane CH activation". J. Organomet. Chem. 689: 4083–4091. doi:10.1016/j.jorganchem.2004.07.034., Lersch, M.Tilset (2005). "Mechanistic Aspects of C−H Activation by Pt Complexes". Chem. Rev. 105: 2471–2526. doi:10.1021/cr030710y., Vedernikov, A. N. (2007). "Recent Advances in the Platinum-mediated CH Bond Functionalization". Curr. Org. Chem. 11: 1401–1416. doi:10.2174/138527207782418708., Davies, H. M. L.; Manning, J. R. (2008). "Catalytic C–H functionalization by metalcarbenoid and nitrenoid insertion". Nature. 451: 417–424. doi:10.1038/nature06485., Boutadla, Y.; Davies, D. L.; Macgregor, S. A.; Poblador-Bahamonde, A. I. "Mechanisms of C–H bond activation: rich synergy between computation and experiment". Dalton Trans. 2009: 5820–5831. doi:10.1039/B904967C., Balcells, D.; Clot, E.; Eisenstein, O. (2010). "C–H Bond Activation in Transition Metal Species from a Computational Perspective". Chem. Rev. 110: 749–823. doi:10.1021/cr900315k., Kuhl, N.; Hopkinson, M. N.; Wencel-Delord, J.; Glorius, F. (2012). "Beyond Directing Groups: Transition Metal-Catalyzed C H Activation of Simple Arenes". Angew. Chem. Int. Ed. 51: 10236–10254. doi:10.1002/anie.201203269., Shulpin, G. B. (2010). "Selectivity enhancement in functionalization of C–H bonds: A review". Org. Biomol. Chem. 8: 4217–4228. doi:10.1039/c004223d.
- 1 2 3 Lyons, T. W.; Sanford, M. S. (2010). "Palladium-Catalyzed Ligand-Directed C–H Functionalization Reactions". Chem. Rev. 110: 1147–1169. doi:10.1021/cr900184e.
- 1 2 Organometallic C–H Bond Activation: An Introduction Alan S. Goldman and Karen I. Goldberg ACS Symposium Series 885, Activation and Functionalization of C–H Bonds, 2004, 1–43
- ↑ Arndtsen, B. A.; Bergman, R. G.; Mobley, T. A.; Peterson, T. H. (1995). "Selective Intermolecular Carbon–Hydrogen Bond Activation by Synthetic Metal Complexes in Homogeneous Solution.". Accounts of Chemical Research. 28 (3): 154–162. doi:10.1021/ar00051a009.
- ↑ Periana, R. A.; Bhalla, G.; Tenn, W. J.; III; Young, K. J. H.; Liu, X. Y.; Mironov, O.; Jones, C.; Ziatdinov, V. R. (2004). "Perspectives on some challenges and approaches for developing the next generation of selective, low temperature, oxidation catalysts for alkane hydroxylation based on the C–H activation reaction". Journal of Molecular Catalysis A: Chemical. 220 (1): 7–25. doi:10.1016/j.molcata.2004.05.036.
- ↑ Wencel-Delord, J.; Glorius, F. (2013). "C–H bond activation enables the rapid construction and late-stage diversification of functional molecules". Nature Chemistry. 5: 369–375. doi:10.1038/nchem.1607.
- ↑ Chatt, J.; Davidson, J. M. "The tautomerism of arene and ditertiary phosphine complexes of ruthenium(0), and the preparation of new types of hydrido-complexes of ruthenium(II)". J. Chem. Soc. 1965: 843. doi:10.1039/JR9650000843.
- ↑ Murahashi, Shunsuke (1955-12-01). "SYNTHESIS OF PHTHALIMIDINES FROM SCHIFF BASES AND CARBON MONOXIDE". Journal of the American Chemical Society. 77 (23): 6403–6404. ISSN 0002-7863. doi:10.1021/ja01628a120.
- ↑ Fekl, U.; Goldberg, K. I. (2003). "Homogeneous Hydrocarbon C-H Bond Activation and Functionalization with Platinum". Advances in Inorganic Chemistry. 54: 259–320. doi:10.1016/S0898-8838(03)54005-3.
- ↑ Fujiwara, Yuzo; Noritani, Ichiro; Danno, Sadao; Asano, Ryuzo; Teranishi, Shiichiro (1969-12-01). "Aromatic substitution of olefins. VI. Arylation of olefins with palladium(II) acetate". Journal of the American Chemical Society. 91 (25): 7166–7169. ISSN 0002-7863. PMID 27462934. doi:10.1021/ja01053a047.
- ↑ Green, M. L.; Knowles, P. J. (1970). "Formation of a tungsten phenyl hydride derivatives from benzene". J. Chem. Soc. D. 24: 1677–1677. doi:10.1039/C29700001677.
- ↑ Foley, Paul; Whitesides, George M. (1979). "Thermal generation of bis(triethylphosphine)-3,3-dimethylplatinacyclobutane from dineopentylbis(triethylphosphine)platinum(II)". J. Am. Chem. Soc. 101 (10): 2732–2733. doi:10.1021/ja00504a041.
- ↑ Janowicz, Andrew H.; Bergman, Robert G. (1982). "Carbon–hydrogen activation in saturated hydrocarbons: direct observation of M + R−H → M(R)(H)". J. Am. Chem. Soc. 104 (1): 352–354. doi:10.1021/ja00365a091.
- ↑ Hoyano, James K.; Graham, William A. G. (1982). "Oxidative addition of the carbon–hydrogen bonds of neopentane and cyclohexane to a photochemically generated iridium(I) complex". J. Am. Chem. Soc. 104 (13): 3723–3725. doi:10.1021/ja00377a032.
- ↑ Baillie, Rhett A.; Legzdins, Peter (2013). "Distinctive Activation and Functionalization of Hydrocarbon C–H Bonds Initiated by Cp*W(NO)(η3-allyl)(CH2CMe3) Complexes". Acc. Chem. Res. 47: ASAP. doi:10.1021/ar400108p.
- ↑ Chetcuti, Michael J.; Ritleng, Vincent (2007). "Formation of a Ruthenium–Arene Complex, Cyclometallation with a Substituted Benzylamine, and Insertion of an Alkyne". J. Chem. Educ. 84: 1014. doi:10.1021/ed084p1014.
- ↑ Murai, Shinji; Kakiuchi, Fumitoshi; Sekine, Shinya; Tanaka, Yasuo; Kamatani, Asayuki; Sonoda, Motohiro; Chatani, Naoto (1993). "Efficient catalytic addition of aromatic carbon–hydrogen bonds to olefins". Nature. 366 (6455): 529–531. Bibcode:1993Natur.366..529M. doi:10.1038/366529a0.
- 1 2 3 4 Brückl, T.; Baxter, R. D.; Ishihara, Y.; Baran, P. S. (2012). "Innate and Guided C-H Functionalization Logic". Accounts of Chemical Research. 45: 826–839. doi:10.1021/ar200194b.
- ↑ Du, X.; Houk, K. N. (1998). "Transition states for Alkane Oxidations by Dioxiranes". J. Org. Chem. 63: 6480–6483. doi:10.1021/jo9801519.
- ↑ Yotphan, Sirilata; Bergman, Robert G.; Ellman, Jonathan A. "The Stereoselective Formation of Bicyclic Enamines with Bridgehead Unsaturation via Tandem C–H Bond Activation/Alkenylation/ Electrocyclization J. Am. Chem. Soc. '". 2008'. 130: 2452–2453. doi:10.1021/ja710981b.
- 1 2 3 Newhouse, T.; Baran, P. S. (2011). "If C-H Bonds Could Talk: Selective C-H Bond Oxidation". Angew. Chem. Int. Ed. 50: 3362–3374. doi:10.1002/anie.201006368.
- ↑ Hass, H. B.; McBee, E. T.; Weber, P. (1936). "Chlorination of Paraffins". Ind. Eng. Chem. 28: 333–339. doi:10.1021/ie50315a017.
- ↑ Wencel-Delord, J.; Dröge, T.; Liu, F.; Glorius, F. (2011). "Towards Mild Metal-Catalyzed C–H Bond Activation". Chem. Soc. Rev. 40: 4740–4761. doi:10.1039/C1CS15083A.
- ↑ Pan, S. C. (2012). "Organocatalytic C–H activation reactions". Beilstein J. Org. Chem. 8: 1374–1384. doi:10.3762/bjoc.8.159.
- ↑ Chen, Huiyuan; Schlecht, Sabine; Semple, Thomas C.; Hartwig, John F. (2000). "Thermal, Catalytic, Regiospecific Functionalization of Alkanes". Science. 287 (5460): 1995–1997. doi:10.1126/science.287.5460.1995.
- ↑ Murphy, J. M.; Lawrence, J. D.; Kawamura, K.; Incarvito, C.; Hartwig, J. F. (2006). "Ruthenium-Catalyzed Regiospecific Borylation of Methyl C-H bonds". J. Am. Chem. Soc. 128: 13684–13685. PMID 17044685. doi:10.1021/ja064092p.
- ↑ Ishiyama, T.; Takagi, J.; Ishida, K.; Miyaura, N.; Anastasi, N. R.; Hartwig, J. F. (2002). "Mild Iridium-Catalyzed Borylation of Arenes. High Turnover Numbers, Room Temperature Reactions, and Isolation of a Potential Intermediate". J. Am. Chem. Soc. 124: 390–391. doi:10.1021/ja0173019.
- ↑ Ishiyama, T.; Takagi, J.; Hartwig, J. F.; Miyaura, N. (2002). "A Stoichiometric Aromatic C-H Borylation Catalyzed by Iridium(I)/2,2′-Bipyridine Complexes at Room Temperature". Angewandte Chemie International Edition. 41: 3056–3058. doi:10.1002/1521-3773(20020816)41:16<3056::aid-anie3056>3.0.co;2-#.
- ↑ Press, L. P.; Kosanovich, A. J.; McCulloch, B. J.; Ozerov, O. V. (2016). "High-Turnover Aromatic C–H Borylation Catalyzed by POCOP-Type Pincer Complexes of Iridium". J. Am. Chem. Soc. 138: 9487–9497. doi:10.1021/jacs.6b03656.
- ↑ Sen, A. (1999). "Catalytic Activation of Methane and Ethane by Metal Compounds". In Murai, S. Activation of Unreactive Bonds and Organic Synthesis. 3. Springer Berlin Heidelberg. pp. 81–95. ISBN 978-3-540-64862-8.
- ↑ "Methanol". www.essentialchemicalindustry.org. Retrieved 2016-02-01.
- ↑ Periana, R.A.; Taube, D.J.; Evitt, E.R.; Loffler, D.G.; Wentrcek, P.R.; Voss, G.; Masuda, T. (1993). "A Mercury-Catalyzed, High-Yield System for the Oxidation of Methane to Methanol". Science. 259 (5093): 340–343. PMID 17832346. doi:10.1126/science.259.5093.340.
- ↑ Periana, R. A.; Taube, D. J.; Gamble, S.; Taube, H.; Satoh, T.; Fujii, H. (1998). "Platinum Catalysts for the High-Yield Oxidation of Methane to a Methanol Derivative". Science. 280 (5363): 560–564. Bibcode:1998Sci...280..560P. PMID 9554841. doi:10.1126/science.280.5363.560.
- ↑ C-H functionalization in Natural Product Total Synthesis Archived December 5, 2014, at the Wayback Machine. C-H Activation: A Complementary Tool in the Total Synthesis of Complex Natural Products
- ↑ O'Malley, S. J.; Tan, K. L.; Watzke, A.; Bergman, R. G.; Ellman, J. A. (2005). "Total Synthesis of (+)-Lithospermic Acid by Asymmetric Intramolecular Alkylation via Catalytic C-H Bond Activation". J. Am. Chem. Soc. 127: 13496–13497. PMID 16190703. doi:10.1021/ja052680h.
- ↑ Ramkumar, N.; Nagarajan, R. (2013). "b. Total Synthesis of Calothrixin A and B via C-H Activation". J. Org. Chem. 78: 2802–2807. doi:10.1021/jo302821v.
- ↑ Ahrendt, Kateri A.; Bergman, Robert G.; Ellman, Jonathan A. (2003-04-01). "Synthesis of a Tricyclic Mescaline Analogue by Catalytic C−H Bond Activation". Organic Letters. 5 (8): 1301–1303. ISSN 1523-7060. PMID 12688744. doi:10.1021/ol034228d.

