C-5 sterol desaturase
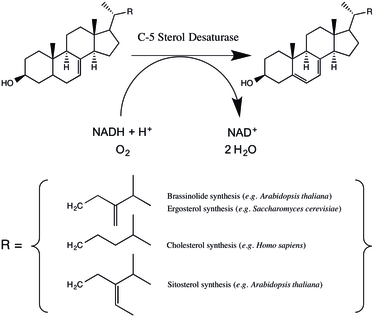
C-5 sterol desaturase (also known as sterol C-5 desaturase and C5SD) is an enzyme that is highly conserved among eukaryotes and catalyzes the dehydrogenation of a C-5(6) bond in a sterol intermediate compound as a step in the biosynthesis of major sterols. The precise structure of the enzyme’s substrate varies by species. For example, the human C-5 sterol desaturase (also known as lathosterol oxidase) oxidizes lathosterol, while its ortholog in the yeast Saccharomyces cerevisiae oxidizes episterol.[1][2]
Mechanism
C-5 sterol desaturase couples sterol oxidation to the oxidation of NAD(P)H and the reduction of molecular oxygen.[2] Either NADH or NADPH can be used; in the model plant species Arabidopsis thaliana C-5 sterol desaturase catalyzes the reaction twice as fast with NADH while in S. cerevisiae the enzyme has little preference.[1][4] The precise details of the reaction have been thought to vary between mammals and yeast.[1] However, the enzymes do share a conserved cluster of histidine resdiues, which when mutated (in A. thaliana) dramatically reduce or eliminate enzyme activity, suggesting the involvement of a coordinated iron cation in the mechanism.[4] Mutagenesis studies suggest that in A. thaliana threonine 114 (which is a serine in humans, mice, and yeast) may help to stabilize the enzyme-substrate complex.[4] Rahier has proposed a reaction mechanism in which an iron-coordinated oxygen abstracts a hydrogen from the substrate leading to a radical intermediate.[5]
Biological role
C-5 sterol desaturase catalyzes an intermediate step in the synthesis of major sterols. The particular biosynthetic pathway varies across eukaryotes. In animals C5SD catalyzes the dehydration of lathosterol to 7-dehydrocholesterol, a step in the synthesis of cholesterol.[6] Cholesterol serves multiple roles in the cell including modulating membrane fluidity serving as a precursor to steroid hormones.[6] In fungi C5SD catalyzes the dehydration of episterol as a step in the synthesis of ergosterol, a sterol that regulates cell membrane fluidity and permeability.[1][7] In plants such as Arabidopsis thaliana, C-5 sterol desaturase catalyzes the dehydrogenation of episterol and avenasterol in a pathway thought to lead to a variety of membrane components as well as a class of hormones called brassinosteroids.[8]
Subcellular localization
Based on its amino acid profile C-5 sterol desaturase appears to have four to five membrane-spanning regions, suggesting that it is a transmembrane protein.[9] C5SD activity has been demonstrated in microsomes from rat tissue, implying that rat enzyme localizes to the endoplasmic reticulum[10][11] Fluorescence microscopy experiments have shown that in the ciliate Tetrahymena thermophila C5SD localizes to the endoplasmic reticulum and that in S. cerevisiae C5SD localizes to both the endoplasmic reticulum and vesicles.[12][13] In Arabidopsis thaliana C5SD is located in both the endoplasmic reticulum and lipid particles.[14]
Clinical Relevance
Antifungal Resistance
The common class of antifungal drugs known as azoles disrupts the fungal sterol biosynthesis pathway, upstream of C-5 sterol desaturase leading to the accumulation of nontoxic 14α-methylated sterols. C5SD then converts these intermediates into a toxic product. Consequently, in both the pathogenic fungus Candida albicans and model organism S. cerevisiae mutations in the gene encoding C-5 sterol desaturase (ERG3) allow the cell to avoid synthesizing the toxic sterol products and have been shown to confer azole resistance.[15][16] In at least the case of fluconazole, antifungal resistance due to C5SD inactivation is dependent on the activity of the chaperone protein Hsp90 and the phosphatase calcineurin.[17][18] However, the clinical relevance of this azole resistance mechanism is controversial because while the deletion of ERG3 alone confers fluconazole resistance to C. albicans in vitro, it is insufficient to confer fluconazole resistance in a live mouse model.[19]
Lathosterolosis
In at least one patient, a deficiency in C-5 sterol desaturase activity (termed lathosterolosis) was associated with multiple malformations, metal retardation, and liver disease.[9] This patient was also found to have low levels of blood cholesterol and high levels of lathosterol in cell membranes when compared to those of healthy control subjects. These symptoms resemble those of other defects in cholesterol synthesis such as Smith–Lemli–Opitz syndrome.[9][20]
Potential applications
Scientists have found that tomato plants engineered with the C-5 sterol desaturase from the mushroom Flammulina velutipes show improved drought tolerance and fungal pathogen resistance as well as increased iron and polyunsaturated fat content.[21] The authors of the study suggest that the fungal enzyme may be a useful tool for plant biotechnology as improving multiple aspects of a crop is typically time- and labor-intensive.
References
- 1 2 3 4 5 Osumi Takashi; Nishino Tokuzo; Katsuki Hirohiko (1979). "Studies on the delta 5-desaturation in ergosterol biosynthesis in yeast". The Journal of Biochemistry. 85 (3): 819–826. PMID 34600.
- 1 2 3 Kawata S, Trzaskos JM, Gaylor JL (1985). "Microsomal enzymes of cholesterol biosynthesis from lanosterol. Purification and characterization of delta 7-sterol 5-desaturase of rat liver microsomes.". Journal of Biological Chemistry. 260 (11): 6609–6617. PMID 3997841.
- ↑ Choe Sunghwa; Noguchi Takahiro; Fujioka Shozo; Takatsuto Suguru; Tissier Christophe P; Gregory Brian D; Ross Amanda S; Tanaka Atsushi; Yoshida Shigeo; Tax Frans E; et al. (1999). "The Arabidopsis dwf7/ste1 mutant is defective in the delta 7 sterol C-5 desaturation step leading to brassinosteroid biosynthesis". The Plant Cell. 11 (2): 207–221. PMC 144158
 . PMID 9927639. doi:10.1105/tpc.11.2.207.
. PMID 9927639. doi:10.1105/tpc.11.2.207. - 1 2 3 Taton Maryse; Husselstein Tania; Benveniste Pierre; Rahier Alain (2000). "Role of highly conserved residues in the reaction catalyzed by recombinant delta 7-sterol-C5 (6)-desaturase studied by site-directed mutagenesis". Biochemistry. 39 (4): 701–711. PMID 10651635. doi:10.1021/bi991467t.
- ↑ Rahier Alain (2001). "Deuterated delta 7-cholestenol analogues as mechanistic probes for wild-type and mutated delta 7-sterol-C5 (6)-desaturase". Biochemistry. 40 (1): 256–267. PMID 11141078. doi:10.1021/bi001696b.
- 1 2 Risley John M (2002). "Cholesterol biosynthesis: Lanosterol to cholesterol". Journal of chemical education. 79 (3): 377. doi:10.1021/ed079p377.
- ↑ Abe Fumiyoshi; Hiraki Toshiki (2009). "Mechanistic role of ergosterol in membrane rigidity and cycloheximide resistance in Saccharomyces cerevisiae". Biochimica et Biophysica Acta (BBA) - Biomembranes. 1788 (3): 743–752. PMID 19118519. doi:10.1016/j.bbamem.2008.12.002.
- ↑ Hartmann Marie-Andrée (1998). "Plant sterols and the membrane environment". Trends in Plant Science. 3 (5): 170–175. doi:10.1016/S1360-1385(98)01233-3.
- 1 2 3 Brunetti-Pierri Nicola; Corso Gaetano; Rossi Massimiliano; Ferrari Paola; Balli Fiorella; Rivasi Francesco; Annunziata Ida; Ballabio Andrea; Russo Antonio Dello; Andria Generoso; et al. (2002). "Lathosterolosis, a novel multiple-malformation/mental retardation syndrome due to deficiency of beta-hydroxysteroid-delta 5-desaturase". The American Journal of Human Genetics. 71 (4): 952–958. PMC 378549
 . PMID 12189593. doi:10.1086/342668.
. PMID 12189593. doi:10.1086/342668. - ↑ Grinstead GF, Gaylor JL (1982). "Total enzymic synthesis of cholesterol from 4, 4, 14 alpha-trimethyl-5 alpha-cholesta-8, 24-dien-3 beta-ol. Solubilization, resolution, and reconstitution of delta 7-sterol 5-desaturase.". The Journal of Biological Chemistry. 257 (23): 13937–44. PMID 6815183.
- ↑ Ishibashi Teruo (2002). "Supernatant protein relevant to the activity of membrane-bound enzymes: studies on lathosterol 5-desaturase". Biochemical and Biophysical Research Communications. 292 (5): 1293–1298. PMID 11969231. doi:10.1006/bbrc.2002.2012.
- ↑ Natter Klaus; Leitner Peter; Faschinger Alexander; Wolinski Heimo; McCraith Stephen; Fields Stanley; Kohlwein Sepp D (2005). "The spatial organization of lipid synthesis in the yeast Saccharomyces cerevisiae derived from large scale green fluorescent protein tagging and high resolution microscopy". Molecular \& Cellular Proteomics. 4 (5): 662–672. PMID 15716577. doi:10.1074/mcp.M400123-MCP200.
- ↑ Poklepovich Tomas J, Rinaldi Mauro A, Tomazic Mariela L, Favale Nicolas O, Turkewitz Aaron P, Nudel Clara B, Nusblat Alejandro D (2012). "The cytochrome b 5 dependent C-5 (6) sterol desaturase DES5A from the endoplasmic reticulum of Tetrahymena thermophila complements ergosterol biosynthesis mutants in Saccharomyces cerevisiae". Steroids. 77 (13): 1313–1320. PMC 3501532
 . PMID 22982564. doi:10.1016/j.steroids.2012.08.015.
. PMID 22982564. doi:10.1016/j.steroids.2012.08.015. - ↑ Silvestro Daniele; Andersen Tonni Grube; Schaller Hubert; Jensen Poul Erik (2013). "Plant sterol metabolism. Delta 7-Sterol-C5-desaturase (STE1/DWARF7), Delta 5, 7-sterol-Delta 7-reductase (DWARF5) and Delta 24-sterol-Delta 24-reductase (DIMINUTO/DWARF1) show multiple subcellular localizations in Arabidopsis thaliana (Heynh) L". PLOS ONE. 8 (2): e56429. PMC 3568079
 . PMID 23409184. doi:10.1371/journal.pone.0056429.
. PMID 23409184. doi:10.1371/journal.pone.0056429. - ↑ Jackson Colin J, Lamb David C, Manning Nigel J, Kelly Diane E, Kelly Steven L (2003). "Mutations in Saccharomyces cerevisiae sterol C5-desaturase conferring resistance to the CYP51 inhibitor fluconazole". Biochemical and Biophysical Research Communications. 309 (4): 999–1004. PMID 13679073. doi:10.1016/j.bbrc.2003.08.098.
- ↑ Vale-Silva LA, Coste AT, Ischer F, Parker JE, Kelly SL, Pinto E, Sanglard D (2012). "Azole resistance by loss of function of the sterol delta 5, 6-desaturase gene (ERG3) in Candida albicans does not necessarily decrease virulence". Antimicrobial Agents and Chemotherapy. 56 (4): 1960–1968. PMC 3318373
 . PMID 22252807. doi:10.1128/AAC.05720-11.
. PMID 22252807. doi:10.1128/AAC.05720-11. - ↑ Cowen Leah E; Lindquist Susan (2005). "Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi". Science. 309 (5744): 2185–2189. PMID 16195452. doi:10.1126/science.1118370.
- ↑ Cowen Leah E; Carpenter Anne E; Matangkasombut Oranart; Fink Gerald R; Lindquist Susan (2006). "Genetic architecture of Hsp90-dependent drug resistance". Eukaryotic cell. 5 (12): 2184–2188. PMC 1694807
 . PMID 17056742. doi:10.1128/EC.00274-06.
. PMID 17056742. doi:10.1128/EC.00274-06. - ↑ Miyazaki Taiga; Miyazaki Yoshitsugu; Izumikawa Koichi; Kakeya Hiroshi; Miyakoshi Shunichi; Bennett John E; Kohno Shigeru (2006). "Fluconazole treatment is effective against a Candida albicans erg3/erg3 mutant in vivo despite in vitro resistance". Antimicrobial Agents and Chemotherapy. 50 (2): 580–586. PMC 1366932
 . PMID 16436713. doi:10.1128/AAC.50.2.580-586.2006.
. PMID 16436713. doi:10.1128/AAC.50.2.580-586.2006.
- ↑ Krakowiak Patrycja A; Wassif Christopher A; Kratz Lisa; Cozma Diana; Kovarova Martina; Harris Ginny; Grinberg Alexander; Yang Yinzi; Hunter Alasdair GW; Tsokos Maria; et al. (2003). "Lathosterolosis: an inborn error of human and murine cholesterol synthesis due to lathosterol 5-desaturase deficiency". Human Molecular Genetics. 12 (13): 1631–1641. PMID 12812989. doi:10.1093/hmg/ddg172.
- ↑ Kamthan Ayushi; Kamthan Mohan; Azam Mohammad; Chakraborty Niranjan; Chakraborty Subhra; Datta Asis (2012). "Expression of a fungal sterol desaturase improves tomato drought tolerance, pathogen resistance and nutritional quality". Scientific Reports. 2: 1–10. PMC 3517979
 . PMID 23230516. doi:10.1038/srep00951.
. PMID 23230516. doi:10.1038/srep00951.