Buparlisib
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| Chemical and physical data | |
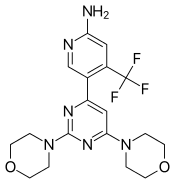
| Formula | C18H21F3N6O2 |
| Molar mass | 410.39 g/mol |
| 3D model (JSmol) | |
| |
Buparlisib (INN,[1] codenamed BKM120) is an investigational small molecule orally-available pan-class I phosphoinositide 3-kinase inhibitor.[2]
Clinical trials
In December 2015 it is reporting results for the phase III BELLE-2 clinical trial for advanced HR+/HER2 endocrine-resistant breast cancer.[3] Encouraging results are reported in some sub-populations — e.g., some PI3K mutations.[3][4]
See also
References
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 68" (PDF). World Health Organization. p. 304. Retrieved 16 April 2016.
- ↑ Geuna, E; Milani, A; Martinello, R; Aversa, C; Valabrega, G; Scaltriti, M; Montemurro, F (March 2015). "Buparlisib, an oral pan-PI3K inhibitor for the treatment of breast cancer". Expert Opinion on Investigational Drugs. 24 (3): 421–31. PMID 25645727. doi:10.1517/13543784.2015.1008132.
- 1 2 PI3K Inhibitor Penetrates Endocrine-Resistant Breast Cancer. Dec 2015
- ↑ Buparlisib Benefits Women With PIK3CA Mutations in Circulating Tumor DNA. Dec 2015
External links
- Buparlisib Shows Promise in Metastatic Breast Cancer. Aug 2014 Buparlisib combined with letrozole
- Phase II Study of Buparlisib + Docetaxel in Advanced or Metastatic Squamous Non-small Cell Lung Cancer (NSCLC) Patients completed
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.