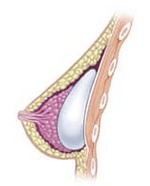
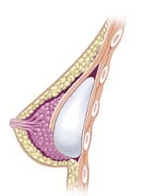
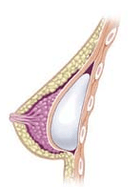
Breast implant

A breast implant is a prosthesis used to change the size, shape, and contour of a woman’s breast. In reconstructive plastic surgery, breast implants can be placed to restore a natural looking breast mound for post–mastectomy breast reconstruction patients or to correct congenital defects and deformities of the chest wall. They are also used cosmetically to enhance or enlarge the appearance of the breast through breast augmentation surgery.
There are three general types of breast implant devices, defined by their filler material: saline solution, silicone gel, and composite filler. The saline implant has an elastomer silicone shell filled with sterile saline solution during surgery; the silicone implant has an elastomer silicone shell pre-filled with viscous silicone gel; and the alternative composition implants featured miscellaneous fillers, such as soy oil, polypropylene string, etc. Composite implants are typically not recommended for use anymore and, in fact, their use is banned in the United States and Europe due to associated health risks and complications.
In surgical practice, for the reconstruction of a breast, the tissue expander device is a temporary breast prosthesis used to form and establish an implant pocket for the future permanent breast implant. For the correction of male breast defects and deformities, the pectoral implant is the breast prosthesis used for the reconstruction and the aesthetic repair of a man’s chest wall (see: gynecomastia and mastopexy).
History

19th century
Since the late nineteenth century, breast implants have been used to surgically augment the size (volume), modify the shape (contour), and enhance the feel (tact) of a woman’s breasts. In 1895, surgeon Vincenz Czerny effected the earliest breast implant emplacement when he used the patient's autologous adipose tissue, harvested from a benign lumbar lipoma, to repair the asymmetry of the breast from which he had removed a tumor.[1] In 1889, surgeon Robert Gersuny experimented with paraffin injections, with disastrous results.
The 20th century
From the first half of the twentieth century, physicians used other substances as breast implant fillers—ivory, glass balls, ground rubber, ox cartilage, Terylene wool, gutta-percha, Dicora, polyethylene chips, Ivalon (polyvinyl alcohol—formaldehyde polymer sponge), a polyethylene sac with Ivalon, polyether foam sponge (Etheron), polyethylene tape (Polystan) strips wound into a ball, polyester (polyurethane foam sponge) Silastic rubber, and teflon-silicone prostheses.[2]
In the mid-twentieth century, Morton I. Berson, in 1945, and Jacques Maliniac, in 1950, each performed flap-based breast augmentations by rotating the patient’s chest wall tissue into the breast to increase its volume. Furthermore, throughout the 1950s and the 1960s, plastic surgeons used synthetic fillers—including silicone injections received by some 50,000 women, from which developed silicone granulomas and breast hardening that required treatment by mastectomy.[3] In 1961, the American plastic surgeons Thomas Cronin and Frank Gerow, and the Dow Corning Corporation, developed the first silicone breast prosthesis, filled with silicone gel; in due course, the first augmentation mammoplasty was performed in 1962 using the Cronin–Gerow Implant, prosthesis model 1963. In 1964, the French company Laboratoires Arion developed and manufactured the saline breast implant, filled with saline solution, and then introduced for use as a medical device in 1964.[4]
Types

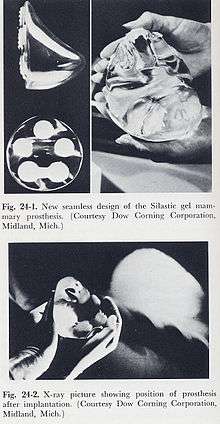
Today, there are two types of breast implants commonly used for mammoplasty, breast reconstruction, and breast augmentation procedures:[5]
- saline implant filled with sterile saline solution.
- silicone implant filled with viscous silicone gel.
Saline implants
The saline breast implant—filled with saline solution (biological-concentration salt water 0.90% w/v of NaCl, ca. 300 mOsm/L.)—was first manufactured by the Laboratoires Arion company, in France, and was introduced for use as a prosthetic medical device in 1964. The contemporary models of saline breast implant are manufactured with thicker, room-temperature vulcanized (RTV) shells made of a silicone elastomer. The study In vitro Deflation of Pre-filled Saline Breast Implants (2006) reported that the rates of deflation (filler leakage) of the pre-filled saline breast implant made it a second-choice prosthesis for corrective breast surgery.[4] Nonetheless, in the 1990s, the saline breast implant was the prosthesis most common device used for breast augmentation surgery in the United States, because of the U.S. FDA’s restriction against the implantation of silicone-filled breast implants outside of clinical studies. Saline breast implants have enjoyed little popularity in the rest of the world, possessing negligible market share.
The technical goal of saline-implant technology was a physically less invasive surgical technique for emplacing an empty breast implant device through a smaller surgical incision.[6] In surgical praxis, after having emplaced the empty breast implants to the implant pockets, the plastic surgeon then filled each device with saline solution, and, because the required insertion-incisions are short and small, the resultant incision-scars will be smaller and shorter than the surgical scars usual to the long incisions required for inserting pre-filled, silicone-gel implants.
When compared to the results achieved with a silicone-gel breast implant, the saline implant can yield acceptable results, of increased breast-size, smoother hemisphere-contour, and realistic texture; yet, it is likelier to cause cosmetic problems, such as the rippling and the wrinkling of the breast-envelope skin, accelerated lower breast pole stretch, and technical problems, such as the presence of the implant being noticeable to the eye and to the touch. The occurrence of such cosmetic problems is likelier in the case of the woman with very little breast tissue, and in the case of the woman who requires post-mastectomy breast reconstruction; thus, the silicone-gel implant is the technically superior prosthetic device for breast augmentation, and for breast reconstruction. In the case of the woman with much breast tissue, for whom sub-muscular emplacement is the recommended surgical approach, saline breast implants can produce an aesthetic result much like that afforded by silicone breast implants, albeit with greater implant palpability.[7]
Silicone gel implants
As a medical device technology, there are five generations of silicone breast implant, each defined by common model-manufacturing techniques.
The modern prosthetic breast was invented in 1961 by the American plastic surgeons Thomas Cronin and Frank Gerow, and manufactured by the Dow Corning Corporation; in due course, the first augmentation mammoplasty was performed in 1962. There are five generations of medical device technology for the breast implant models filled with silicone gel; each generation of breast prosthesis is defined by common model-manufacturing techniques.
First generation
The Cronin–Gerow Implant, prosthesis model 1963, was a silicone rubber envelope-sac, shaped like a teardrop, which was filled with viscous silicone-gel. To reduce the rotation of the emplaced breast implant upon the chest wall, the model 1963 prosthesis was affixed to the implant pocket with a fastener-patch, made of Dacron material (Polyethylene terephthalate), which was attached to the rear of the breast implant shell.[8]
Second generation
In the 1970s, manufacturers presented the second generation of breast implant prostheses that featured functional developments and aesthetic improvements to the technology:
- the first technological developments were a thinner-gauge device-shell, and a filler gel of low-cohesion silicone, which improved the functionality and the verisimilitude (size, appearance, and texture) of the silicone-gel breast implant. Yet, in clinical practice, second-generation breast implants proved fragile, and suffered greater incidences of shell rupture, and of filler leakage ("silicone-gel bleed") through the intact device shell. The consequent, increased incidence-rates of medical complications (e.g. capsular contracture) precipitated faulty-product, class action-lawsuits, by the U.S. government, against the Dow Corning Corporation, and other manufacturers of breast prostheses.
- the second technological development was a polyurethane foam coating for the shell of the breast implant; the coating reduced the incidence of capsular contracture, by causing an inflammatory reaction that impeded the formation of a capsule of fibrous collagen tissue around the breast implant. Nevertheless, despite that prophylactic measure, the medical use of polyurethane-coated breast implants was briefly discontinued, because of the potential health-risk posed by 2,4-toluenediamine (TDA), a carcinogenic by-product of the chemical breakdown of the polyurethane foam coating of the breast implant.[9]
- After reviewing the medical data, the U.S. Food and Drug Administration concluded that TDA-induced breast cancer was an infinitesimal health-risk to women with breast implants, and did not justify legally requiring physicians to explain the matter to their patients. In the event, polyurethane-coated breast implants remain in plastic surgery practice in Europe and in South America; and no manufacturer has sought FDA approval for medical sales of such breast implants in the U.S.[10]
- the third technological development was the double lumen breast implant device, a double-cavity prosthesis composed of a silicone breast implant contained within a saline breast implant. The two-fold, technical goal was: (i) the cosmetic benefits of silicone-gel (the inner lumen) enclosed in saline solution (the outer lumen); (ii) a breast implant device the volume of which is post-operatively adjustable. Nevertheless, the more complex design of the double-lumen breast implant suffered a device-failure rate greater than that of single-lumen breast implants. The contemporary versions of second-generation breast implant devices (presented in 1984) are the "Becker Expandable" models of breast implant, which are primarily used for breast reconstruction.
Third and Fourth generations
In the 1980s, the models of the Third and of the Fourth generations of breast implant devices were sequential advances in manufacturing technology, such as elastomer-coated shells that decreased gel-bleed (filler leakage), and a thicker (increased-cohesion) filler gel. Sociologically, the manufacturers of prosthetic breasts then designed and made anatomic models (natural breast) and shaped models (round, tapered) that realistically corresponded with the breast- and body- types of women. The tapered models of breast implant have a uniformly textured surface, which reduces the rotation of the prosthesis within the implant pocket; the round models of breast implant are available in smooth-surface- and textured-surface- types.
Fifth generation
Since the mid-1990s, the fifth generation of silicone-gel breast implant is made of a high-strength, highly cohesive silicone gel that mostly eliminates the occurrences of filler leakage (“silicone gel bleed”) and of the migration of the silicone filler from the implant pocket to elsewhere in the woman’s body. These implants are commonly referred to as "gummy bear breast implants" for their firm, pliant consistency, which is similar to gummy candies. The studies Experience with Anatomical Soft Cohesive Silicone gel Prosthesis in Cosmetic and Reconstructive Breast Implant Surgery (2004) and Cohesive Silicone gel Breast Implants in Aesthetic and Reconstructive Breast Surgery (2005) reported low incidence-rates of capsular contracture and of device-shell rupture; and greater rates of improved medical-safety and technical-efficacy than that of early generation breast implant devices.[11][12][13]
Psychology
The breast augmentation patient usually is a young woman whose personality profile indicates psychological distress about her personal appearance and her bodily self image, and a history of having endured criticism (teasing) about the aesthetics of her person.[14] The studies Body Image Concerns of Breast Augmentation Patients (2003) and Body Dysmorphic Disorder and Cosmetic Surgery (2006) reported that the woman who underwent breast augmentation surgery also had undergone psychotherapy, suffered low self-esteem, presented frequent occurrences of psychological depression, had attempted suicide, and suffered body dysmorphia, a type of mental illness.
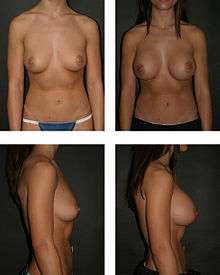
Post-operative patient surveys about mental health and quality-of-life, reported improved physical health, physical appearance, social life, self-confidence, self-esteem, and satisfactory sexual functioning. Furthermore, the women reported long-term satisfaction with their breast implant outcomes; some despite having suffered medical complications that required surgical revision, either corrective or aesthetic. Likewise, in Denmark, 8.0 per cent of breast augmentation patients had a pre-operative history of psychiatric hospitalization.[15][16][17][18][19][20][21][22][23]
In 2008, the longitudinal study Excess Mortality from Suicide and other External Causes of Death Among Women with Cosmetic Breast Implants (2007), reported that women who sought breast implants are almost 3.0 times as likely to commit suicide as are women who have not sought breast implants. Compared to the standard suicide-rate for women of the general populace, the suicide-rate for women with augmented breasts remained constant until 10-years post-implantation, yet, it increased to 4.5 times greater at the 11-year mark, and so remained until the 19-year mark, when it increased to 6.0 times greater at 20-years post-implantation. Moreover, additional to the suicide-risk, women with breast implants also faced a trebled death-risk from alcoholism and the abuse of prescription and recreational drugs.[24][25] Although seven studies have statistically connected a woman’s breast augmentation to a greater suicide-rate, the research indicates that breast augmentation surgery does not increase the death rate; and that, in the first instance, it is the psychopathologically-inclined woman who is more likely to undergo a breast augmentation procedure.[26][27][28][29][30][31]
The study Effect of Breast Augmentation Mammoplasty on Self-Esteem and Sexuality: A Quantitative Analysis (2007), reported that the women attributed their improved self image, self-esteem, and increased, satisfactory sexual functioning to having undergone breast augmentation; the cohort, aged 21–57 years, averaged post-operative self-esteem increases that ranged from 20.7 to 24.9 points on the 30-point Rosenberg self-esteem scale, which data supported the 78.6 per cent increase in the woman’s libido, relative to her pre-operative level of libido.[32] Therefore, before agreeing to any surgery, the plastic surgeon evaluates and considers the woman’s mental health to determine if breast implants can positively affect her self-esteem and sexual functioning.
Surgical procedures
Indications
A mammoplasty procedure for the placement of breast implant devices has three (3) purposes:
- primary reconstruction: the replacement of breast tissues damaged by trauma (blunt, penetrating, blast), disease (breast cancer), and failed anatomic development (tuberous breast deformity).
- revision and reconstruction: to revise (correct) the outcome of a previous breast reconstruction surgery.
- primary augmentation: to aesthetically augment the size, form, and feel of the breasts.
The operating room (OR) time of post–mastectomy breast reconstruction, and of breast augmentation surgery is determined by the procedure employed, the type of incisions, the breast implant (type and materials), and the pectoral locale of the implant pocket.
Recent research has indicated that mammograms should not be done with any increased frequency than used in normal procedure in patients undergoing breast surgery, including breast implant, augmentation, mastopexy, and breast reducation.[33]
Incision types
Breast implant emplacement is performed with five (5) types of surgical incisions:
- Inframammary: an incision made to the inframammary fold (natural crease under your breast), which affords maximal access for precise dissection of the tissues and emplacement of the breast implants. It is the preferred surgical technique for emplacing silicone-gel implants, because it better exposes the breast tissue–pectoralis muscle interface; yet, IMF implantation can produce thicker, slightly more visible surgical scars.
- Periareolar: a border-line incision along the periphery of the areola, which provides an optimal approach when adjustments to the IMF position are required, or when a mastopexy (breast lift) is included to the primary mammoplasty procedure. In periareolar emplacement, the incision is around the medial-half (inferior half) of the areola’s circumference. Silicone gel implants can be difficult to emplace via periareolar incision, because of the short, five-centimetre length (~ 5.0 cm) of the required access-incision. Aesthetically, because the scars are at the areola’s border (periphery), they usually are less visible than the IMF-incision scars of women with light-pigment areolae; when compared to cutaneous-incision scars, the modified epithelia of the areolae are less prone to (raised) hypertrophic scars.
- Transaxillary: an incision made to the axilla (armpit), from which the dissection tunnels medially, to emplace the implants, either bluntly or with an endoscope (illuminated video microcamera), without producing visible scars on the breast proper; yet, it is likelier to produce inferior asymmetry of the implant-device position. Therefore, surgical revision of transaxillary emplaced breast implants usually requires either an IMF incision or a periareolar incision.
- Transumbilical: a trans-umbilical breast augmentation (TUBA) is a less common implant-device emplacement technique wherein the incision is at the umbilicus (navel), and the dissection tunnels superiorly, up towards the bust. The TUBA approach allows emplacing the breast implants without producing visible scars upon the breast proper; but makes appropriate dissection and device-emplacement more technically difficult. A TUBA procedure is performed bluntly—without the endoscope’s visual assistance—and is not appropriate for emplacing (pre-filled) silicone-gel implants, because of the great potential for damaging the elastomer silicone shell of the breast implant during its manual insertion through the short (~2.0 cm) incision at the navel, and because pre-filled silicone gel implants are incompressible, and cannot be inserted through so small an incision.[34]
- Transabdominal: as in the TUBA procedure, in the transabdominoplasty breast augmentation (TABA), the breast implants are tunneled superiorly from the abdominal incision into bluntly dissected implant pockets, whilst the patient simultaneously undergoes an abdominoplasty.[35]
Implant pocket placement
The four surgical approaches to emplacing a breast implant to the implant pocket are described in anatomical relation to the pectoralis major muscle.
- Subglandular: the breast implant is emplaced to the retromammary space, between the breast tissue (the mammary gland) and the pectoralis major muscle (major muscle of the chest), which most approximates the plane of normal breast tissue, and affords the most aesthetic results. Yet, in women with thin pectoral soft-tissue, the subglandular position is likelier to show the ripples and wrinkles of the underlying implant. Moreover, the capsular contracture incidence rate is slightly greater with subglandular implantation.
- Subfascial: the breast implant is emplaced beneath the fascia of the pectoralis major muscle; the subfascial position is a variant of the subglandular position for the breast implant.[36] The technical advantages of the subfascial implant-pocket technique are debated; proponent surgeons report that the layer of fascial tissue provides greater implant coverage and better sustains its position.[37]
- Subpectoral (dual plane): the breast implant is emplaced beneath the pectoralis major muscle, after the surgeon releases the inferior muscular attachments, with or without partial dissection of the subglandular plane. Resultantly, the upper pole of the implant is partially beneath the pectoralis major muscle, while the lower pole of the implant is in the subglandular plane. This implantation technique achieves maximal coverage of the upper pole of the implant, whilst allowing the expansion of the implant’s lower pole; however, “animation deformity”, the movement of the implants in the subpectoral plane can be excessive for some patients.[38]
- Submuscular: the breast implant is emplaced beneath the pectoralis major muscle, without releasing the inferior origin of the muscle proper. Total muscular coverage of the implant can be achieved by releasing the lateral muscles of the chest wall—either the serratus muscle or the pectoralis minor muscle, or both—and suturing it, or them, to the pectoralis major muscle. In breast reconstruction surgery, the submuscular implantation approach effects maximal coverage of the breast implants. This technique is rarely used in cosmetic surgery due to high risk of animation deformities.
Post-surgical recovery
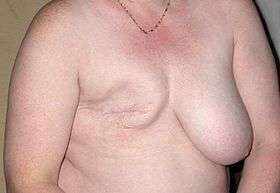
The surgical scars of a breast augmentation mammoplasty develop approximately at 6-weeks post-operative, and fade within months. Depending upon the daily-life physical activities required of the woman, the breast augmentation patient usually resumes her normal life at 1-week post-operative. Moreover, women whose breast implants were emplaced beneath the chest muscles (submuscular placement) usually have a longer, slightly more painful convalescence, because of the healing of the incisions to the chest muscles. Usually, she does not exercise or engage in strenuous physical activities for approximately 6 weeks. During the initial post-operative recovery, the woman is encouraged to regularly exercise (flex and move) her arm to alleviate pain and discomfort; if required, analgesic indwelling medication catheters can alleviate pain[39][40] Moreover, significantly improved patient recovery has resulted from refined breast-device implantation techniques (submuscular, subglandular) that allow 95 per cent of women to resume their normal lives at 24-hours post-procedure, without bandages, fluid drains, pain pumps, catheters, medical support brassières, or narcotic pain medication.[41][42][43][44]
Complications
The plastic surgical emplacement of breast implant devices, either for breast reconstruction or for aesthetic purpose, presents the same health risks common to surgery, such as adverse reaction to anesthesia, hematoma (post-operative bleeding), late hematoma (post-operative bleeding after 6 months or more),[45] seroma (fluid accumulation), incision-site breakdown (wound infection). Complications specific to breast augmentation include breast pain, altered sensation, impeded breast-feeding function, visible wrinkling, asymmetry, thinning of the breast tissue, and symmastia, the “bread loafing” of the bust that interrupts the natural plane between the breasts. Specific treatments for the complications of indwelling breast implants—capsular contracture and capsular rupture—are periodic MRI monitoring and physical examinations. Furthermore, complications and re-operations related to the implantation surgery, and to tissue expanders (implant place-holders during surgery) can cause unfavorable scarring in approximately 6–7 per cent of the patients. [46][47][48] Statistically, 20 per cent of women who underwent cosmetic implantation, and 50 per cent of women who underwent breast reconstruction implantation, required their explantation at the 10-year mark.[49]
Implant rupture
Because a breast implant is a Class III medical device of limited product-life, the principal rupture-rate factors are its age and design; Nonetheless, a breast implant device can retain its mechanical integrity for decades in a woman’s body.[50] When a saline breast implant ruptures, leaks, and empties, it quickly deflates, and thus can be readily explanted (surgically removed). The follow-up report, Natrelle Saline-filled Breast Implants: a Prospective 10-year Study (2009) indicated rupture-deflation rates of 3–5 per cent at 3-years post-implantation, and 7–10 per cent rupture-deflation rates at 10-years post-implantation.[51]
When a silicone breast implant ruptures it usually does not deflate, yet the filler gel does leak from it, which can migrate to the implant pocket; therefore, an intracapsular rupture (in-capsule leak) can become an extracapsular rupture (out-of-capsule leak), and each occurrence is resolved by explantation. Although the leaked silicone filler-gel can migrate from the chest tissues to elsewhere in the woman’s body, most clinical complications are limited to the breast and armpit areas, usually manifested as granulomas (inflammatory nodules) and axillary lymphadenopathy (enlarged lymph glands in the armpit area).[52][53][54]
The suspected mechanisms of breast implant rupture are:
- damage during implantation
- damage during (other) surgical procedures
- chemical degradation of the breast implant shell
- trauma (blunt trauma, penetrating trauma, blast trauma)
- mechanical pressure of traditional mammographic breast examination [55]
Silicone implant rupture can be evaluated using magnetic resonance imaging; from the long-term MRI data for single-lumen breast implants, the European literature about second generation silicone-gel breast implants (1970s design), reported silent device-rupture rates of 8–15 per cent at 10-years post-implantation (15–30% of the patients).[56][57][58][59]
The study Safety and Effectiveness of Mentor’s MemoryGel Implants at 6 Years (2009), which was a branch study of the U.S. FDA’s core clinical trials for primary breast augmentation surgery patients, reported low device-rupture rates of 1.1 per cent at 6-years post-implantation.[60] The first series of MRI evaluations of the silicone breast implants with thick filler-gel reported a device-rupture rate of 1.0 per cent, or less, at the median 6-year device-age.[61] Statistically, the manual examination (palpation) of the woman is inadequate for accurately evaluating if a breast implant has ruptured. The study, The Diagnosis of Silicone Breast implant Rupture: Clinical Findings Compared with Findings at Magnetic Resonance Imaging (2005), reported that, in asymptomatic patients, only 30 per cent of the ruptured breast implants are accurately palpated and detected by an experienced plastic surgeon, whereas MRI examinations accurately detected 86 per cent of breast implant ruptures.[62] Therefore, the U.S. FDA recommended scheduled MRI examinations, as silent-rupture screenings, beginning at the 3-year-mark post-implantation, and then every two years, thereafter.[46] Nonetheless, beyond the U.S., the medical establishments of other nations have not endorsed routine MRI screening, and, in its stead, proposed that such a radiologic examination be reserved for two purposes: (i) for the woman with a suspected breast implant rupture; and (ii) for the confirmation of mammographic and ultrasonic studies that indicate the presence of a ruptured breast implant.[63]
Furthermore, The Effect of Study design Biases on the Diagnostic Accuracy of Magnetic Resonance Imaging for Detecting Silicone Breast Implant Ruptures: a Meta-analysis (2011) reported that the breast-screening MRIs of asymptomatic women might overestimate the incidence of breast implant rupture.[64] In the event, the U.S. Food and Drug Administration emphasised that “breast implants are not lifetime devices. The longer a woman has silicone gel-filled breast implants, the more likely she is to experience complications.”[65]
Capsular contracture
The human body’s immune response to a surgically installed foreign object—breast implant, cardiac pacemaker, orthopedic prosthesis—is to encapsulate it with scar tissue capsules of tightly woven collagen fibers, in order to maintain the integrity of the body by isolating the foreign object, and so tolerate its presence. Capsular contracture—which should be distinguished from normal capsular tissue—occurs when the collagen-fiber capsule thickens and compresses the breast implant; it is a painful complication that might distort either the breast implant, or the breast, or both.
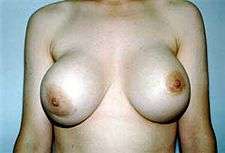
The cause of capsular contracture is unknown, but the common incidence factors include bacterial contamination, device-shell rupture, filler leakage, and hematoma. The surgical implantation procedures that have reduced the incidence of capsular contracture include submuscular emplacement, the use of breast implants with a textured surface (polyurethane-coated);[66][67][68] limited pre-operative handling of the implants, limited contact with the chest skin of the implant pocket before the emplacement of the breast implant, and irrigation of the recipient site with triple-antibiotic solutions.[69][70]
The correction of capsular contracture might require an open capsulotomy (surgical release) of the collagen-fiber capsule, or the removal, and possible replacement, of the breast implant. Furthermore, in treating capsular contracture, the closed capsulotomy (disruption via external manipulation) once was a common maneuver for treating hard capsules, but now is a discouraged technique, because it can rupture the breast implant. Non-surgical treatments for collagen-fiber capsules include massage, external ultrasonic therapy, leukotriene pathway inhibitors such as zafirlukast (Accolate) or montelukast (Singulair), and pulsed electromagnetic field therapy (PEMFT).[71][72][73][74]
Repair and revision surgeries
When the patient is unsatisfied with the outcome of the augmentation mammoplasty; or when technical or medical complications occur; or because of the breast implants’ limited product life, it is likely she might require replacing the breast implants. Common revision surgery indications include major and minor medical complications, capsular contracture, shell rupture, and device deflation.[55] Revision incidence rates were greater for breast reconstruction patients, because of the post-mastectomy changes to the soft-tissues and to the skin envelope of the breast, and to the anatomical borders of the breast, especially in women who received adjuvant external radiation therapy.[55] Moreover, besides breast reconstruction, breast cancer patients usually undergo revision surgery of the nipple-areola complex (NAC), and symmetry procedures upon the opposite breast, to create a bust of natural appearance, size, form, and feel. Carefully matching the type and size of the breast implants to the patient’s pectoral soft-tissue characteristics reduces the incidence of revision surgery. Appropriate tissue matching, implant selection, and proper implantation technique, the re-operation rate was 3.0 per cent at the 7-year-mark, compared with the re-operation rate of 20 per cent at the 3-year-mark, as reported by the U.S. Food and Drug Administration.[75][76]
Systemic Illness and ALCL Cancer
Systemic disease and sickness
Many women with breast implants have reported connective tissue diseases such as systemic sclerosis, systemic lupus erythematosus, rheumatoid arthritis, and fibromyalgia. Various other systemic symptoms have been evidenced at large and fall under the term Breast Implant Illness. Symptoms include extreme fatigue, cognitive dysfunction (brain fog, memory loss, difficulty concentrating), joint and muscle pain, dryness throughout the body, hair loss, tingling and numbness in the extremities, recurring infections, endocrine problems (thyroid, adrenals, etc.), and more.[77][78][79][80][81][82]
Platinum toxicity
Platinum is used in the production of silicone gel for breast implants and small amounts may leach into surrounding tissues. Dr. Ernest Lykissa and Dr. Susan Maharaj did research on silicone breast implants and platinum. In their Analytical Chemistry paper, which is a top journal of the field, they reported finding the highest platinum to date in women who had implants. They also found that the platinum is transformed into an oxidized state which is more harmful.[83][84][85][86]
Anaplastic large cell lymphoma
The FDA has identified that breast implants may cause a rare form of cancer called anaplastic large-cell lymphoma, notably when the implants are textured.[87] As of February 1, 2017, the FDA has received a total of 359 medical device reports of breast-implant-associated ALCL, including 9 deaths.[88] If women have swelling or fluid collection, a CD30 test must be done. The American Society of Plastic Surgery (ASPS) states, "CD30 is the main diagnostic test that must be performed on the seroma fluid as routine pathology or H&E staining can frequently miss the diagnosis." [89] Diagnosis and treatment of breast implant associated ALCL now follows standardized guidelines established by the National Comprehensive Cancer Network.[90]
"The current lifetime risk of BIA-ALCL in the U.S. is estimated to be 1:30,000 women with textured implants based upon current confirmed cases and textured implant sales data over the past two decades. This is consistent with risk reported in Europe.Certain geographic locations have demonstrated variable risks. For instance, a December 2016 update from the Therapeutic Goods Administration of Australia and New Zealand reported a risk of 1:1,000 to 1:10,000 for textured implants." [89]
The ASPS and the Plastic Surgery Foundation (PSF) have partnered with the FDA to study this condition and in doing so created the Patient Registry and Outcomes For breast Implants and anaplastic large cell Lymphoma Etiology and epidemiology (PROFILE). The United States FDA strongly encourages all physicians to report cases to PROFILE in an effort to better understand the role of breast implants in ALCL and the management of this disease.[91]
Implants and breast-feeding


Digestive contamination and systemic toxicity are the principal infant-health concerns; the leakage of breast implant filler to the breast milk, and if the filler is dangerous to the nursing infant. Breast implant device fillers are biologically inert—saline filler is salt water, and silicone filler is indigestible—because each substance is chemically inert, and environmentally common. Moreover, proponent physicians have said there “should be no absolute contraindication to breast-feeding by women with silicone breast implants.”[92] In the early 1990s, at the beginning of the silicone breast implant sickness occurrences, small-scale, non-random studies (i.e. “patients came with complaints, which might have many sources”, not “doctors performed random tests”) indicated possible breast-feeding complications from silicone implants; yet no study reported device–disease causality.[93]
Women with breast implants may have functional breast-feeding difficulties; mammoplasty procedures that feature periareolar incisions are especially likely to cause breast-feeding difficulties. Surgery may also damage the lactiferous ducts and the nerves in the nipple-areola area.[94][95][96]
Functional breast-feeding difficulties arise if the surgeon cut the milk ducts or the major nerves innervating the breast, or if the milk glands were otherwise damaged. Milk duct and nerve damage are more common if the incisions cut tissue near the nipple. The milk glands are most likely to be affected by subglandular implants (under the gland), and by large-sized breast implants, which pinch the lactiferous ducts and impede milk flow. Small-sized breast implants, and submuscular implantation, cause fewer breast-function problems; however, it is impossible to predict whether a woman who undergoes breast augmentation will be able to successfully breast feed since some women are able to breast-fed after periareolarincisions and subglandular placement and some are not able to after augmentation using submuscular and other types of surgical incisions.[96]
Implants and mammography
The presence of radiologically opaque breast implants (either saline or silicone) might interfere with the radiographic sensitivity of the mammograph, that is, the image might not show any tumor(s) present. In which case, anEklund view mammogram is required to ascertain either the presence or the absence of a cancerous tumor, wherein the breast implant is manually displaced against the chest wall and the breast is pulled forward, so that the mammograph can visualize a greater volume of the internal tissues; Nonetheless, approximately one-third of the breast tissue remains inadequately visualized, resulting in an increased incidence of mammograms with false-negative results.[97]

The breast cancer studies Cancer in the Augmented Breast: Diagnosis and Prognosis (1993) and Breast Cancer after Augmentation Mammoplasty (2001) of women with breast implant prostheses reported no significant differences in disease-stage at the time of the diagnosis of cancer; prognoses are similar in both groups of women, with augmented patients at a lower risk for subsequent cancer recurrence or death.[98][99] Conversely, the use of implants for breast reconstruction after breast cancer mastectomy appears to have no negative effect upon the incidence of cancer-related death.[100] That patients with breast implants are more often diagnosed with palpable—but not larger—tumors indicates that equal-sized tumors might be more readily palpated in augmented patients, which might compensate for the impaired mammogram images.[101] The ready palpability of the breast-cancer tumor(s) is consequent to breast tissue thinning by compression, innately in smaller breasts a priori (because they have lesser tissue volumes), and that the implant serves as a radio-opaque base against which a cancerous tumor can be differentiated.[102]
The breast implant has no clinical bearing upon lumpectomy breast-conservation surgery for women who developed breast cancer after the implantation procedure, nor does the breast implant interfere with external beam radiation treatments (XRT); moreover, the post-treatment incidence of breast-tissue fibrosis is common, and thus a consequent increased rate of capsular contracture.[103] The study Breast Cancer Detection and Survival among Women with Cosmetic Breast Implants: Systematic Review and Meta-analysis of Observational Studies, reported a significant delay in the diagnoses of women who developed breast cancer after undergoing breast augmentation, when compared to breast cancer patients who had not undergone breast augmentation.[104] The metadata study Breast Implants following Mastectomy in Women with Early-stage Breast Cancer: Prevalence and Impact on Survival (2005) reported that breast augmentation patients were statistically likelier to die from breast cancer. Although the use of implants for breast reconstruction after breast cancer mastectomy appears to have no negative effect upon the incidence of cancer-related death, women who underwent a mastectomy procedure tend to die earlier than women who underwent a lumpectomy procedure, with like diagnoses.[100][105]
U.S. FDA approval
In 1988, twenty-six years after the 1962 introduction of breast implants filled with silicone gel, the U.S. Food and Drug Administration (FDA) investigated breast implant failures and the subsequent complications, and re-classified breast implant devices as Class III medical devices, and required from manufacturers the documentary data substantiating the safety and efficacy of their breast implant devices.[106] In 1992, the FDA placed silicone-gel breast implants in moratorium in the U.S., because there was “inadequate information to demonstrate that breast implants were safe and effective”. Nonetheless, medical access to silicone-gel breast implant devices continued for clinical studies of post-mastectomy breast reconstruction, the correction of congenital deformities, and the replacement of ruptured silicone-gel implants. The FDA required from the manufacturers the clinical trial data, and permitted their providing breast implants to the breast augmentation patients for the statistical studies required by the U.S. Food and Drug Administration.[106] In mid–1992, the FDA approved an adjunct study protocol for silicone-gel filled implants for breast reconstruction patients, and for revision-surgery patients. Also in 1992, the Dow Corning Corporation, a silicone products and breast implant manufacturer, announced the discontinuation of five implant-grade silicones, but would continue producing 45 other, medical-grade, silicone materials—three years later, in 1995, the Dow Corning Corporation went bankrupt when it faced 19,000 breast implant sickness lawsuits.[106]
- In 1997, the U.S. Department of Health and Human Services (HHS) appointed the Institute of Medicine (IOM) of the U.S. National Academy of Sciences (NAS) to investigate the potential risks of operative and post-operative complications from the emplacement of silicone breast implants. The IOM’s review of the safety and efficacy of silicone gel-filled breast implants, reported that the "evidence suggests diseases or conditions, such as connective tissue diseases, cancer, neurological diseases, or other systemic complaints or conditions are no more common in women with breast implants, than in women without implants" subsequent studies and systemic review found no causal link between silicone breast implants and disease.[106]

- In 1998, the U.S. FDA approved adjunct study protocols for silicone-gel filled implants only for breast reconstruction patients and for revision-surgery patients; and also approved the Dow Corning Corporation’s Investigational Device Exemption (IDE) study for silicone-gel breast implants for a limited number of breast augmentation-, reconstruction-, and revision-surgery patients.[106]
- In 1999, the Institute of Medicine published the Safety of Silicone Breast Implants (1999) study that reported no evidence that saline-filled and silicone-gel filled breast implant devices caused systemic health problems; that their use posed no new health or safety risks; and that local complications are “the primary safety issue with silicone breast implants”, in distinguishing among routine and local medical complications and systemic health concerns.”[106][107][108]
- In 2000, the FDA approved saline breast implant Premarket Approval Applications (PMA) containing the type and rate data of the local medical complications experienced by the breast surgery patients.[109] "Despite complications experienced by some women, the majority of those women still in the Inamed Corporation and Mentor Corporation studies, after three years, reported being satisfied with their implants."[106] The premarket approvals were granted for breast augmentation, for women at least 18 years old, and for women requiring breast reconstruction.[110][111]
- In 2006, for the Inamed Corporation and for the Mentor Corporation, the U.S. Food and Drug Administration lifted its restrictions against using silicone-gel breast implants for breast reconstruction and for augmentation mammoplasty. Yet, the approval was conditional upon accepting FDA monitoring, the completion of 10-year-mark studies of the women who already had the breast implants, and the completion of a second, 10-year-mark study of the safety of the breast implants in 40,000 other women.[112] The FDA warned the public that breast implants do carry medical risks, and recommended that women who undergo breast augmentation should periodically undergo MRI examinations to screen for signs of either shell rupture or of filler leakage, or both conditions; and ordered that breast surgery patients be provided with detailed, informational brochures explaining the medical risks of using silicone-gel breast implants.[106]
The U.S. Food and Drug Administration established the age ranges for women seeking breast implants; for breast reconstruction, silicone-gel filled implants and saline-filled implants were approved for women of all ages; for breast augmentation, saline implants were approved for women 18 years of age and older; silicone implants were approved for women 22 years of age and older.[113] Because each breast implant device entails different medical risks, the minimum age of the patient for saline breast implants is different from the minimum age of the patient for silicone breast implants—because of the filler leakage and silent shell-rupture risks; thus, periodic MRI screening examinations are the recommended post-operative, follow-up therapy for the patient.[114] In other countries, in Europe and Oceania, the national health ministries' breast implant policies do not endorse periodic MRI screening of asymptomatic patients, but suggest palpation proper—with or without an ultrasonic screening—to be sufficient post-operative therapy for most patients.
Criticism
In the early 1990s, the national health ministries of the listed countries reviewed the pertinent studies for causal links among silicone-gel breast implants and systemic and auto-immune diseases. The collective conclusion is that there is no evidence establishing a causal connection between the implantation of silicone breast implants and either type of disease. The affected women complained of systemic disease manifested as fungal, neurologic, and rheumatologic ailments. The Danish study Long-term Health Status of Danish Women with Silicone Breast Implants (2004) reported that women who had breast implants for an average of 19 years were no more likely to report an excessive number of rheumatic disease symptoms than would the women of the control group.[115] The follow-up study Mortality Rates Among Augmentation Mammoplasty Patients: An Update (2006) reported a decreased standardized mortality ratio and an increased risk of lung cancer death among breast implant patients, than among patients for other types of plastic surgery; the mortality rate differences were attributed to tobacco smoking.[116] The study Mortality Among Canadian Women with Cosmetic Breast Implants (2006), about some 25,000 women with breast implants, reported a 43 per cent lower rate of breast cancer among them than among the general populace, and a lower-than-average risk of cancer.[117]
| Year | Country | Systemic Review Group | Conclusions |
|---|---|---|---|
| 1991–93 | United Kingdom | Independent Expert Advisory Group (IEAG) | There was no evidence of an increased risk of connective-tissue disease in patients who had undergone silicone-gel breast implant emplacement, and no cause for changing either breast implant practice or policy in the U.K. |
| 1996 | United States | U.S. Institute of Medicine (IOM)[118] | There was "insufficient evidence for an association of silicone gel- or saline-filled breast implants with defined connective tissue disease." |
| 1996 | France | Agence Nationale pour le Developpement de l’Evaluation Medicale (ANDEM) [National Agency for Medical Development and Evaluation][119] | French original: "Nous n'avons pas observé de connectivité ni d'autre pathologie auto-immune susceptible d'être directement ou indirectement induite par la présence d'un implant mammaire en particulier en gel de silicone...."
English translation: "We did not observe connective tissue diseases to be directly or indirectly associated by the presence of a breast implant, in particular one of silicone gel...." |
| 1997 | Australia | Therapeutic Devices Evaluation Committee (TDEC) | The "current, high-quality literature suggest that there is no association between breast implants and connective tissue disease-like syndromes (atypical connective tissue diseases)."[120] |
| 1998 | Germany | Federal Institute for Medicine and Medical Products | Reported that "silicone breast implants neither cause auto-immune diseases nor rheumatic diseases and have no disadvantageous effects on pregnancy, breast-feeding capability, or the health of children who are breast-fed. There is no scientific evidence for the existence of silicone allergy, silicone poisoning, atypical silicone diseases or a new silicone disease."[121] |
| 2000 | United States | Federal court-ordered review[122] | "No evidence of an association between... silicone-gel-filled breast implants specifically, and any of the individual CTDs, all definite CTDs combined, or other auto-immune or rheumatic conditions." |
| 2000 | European Union | European Committee on Quality Assurance & Medical Devices in Plastic Surgery (EQUAM) | "Additional medical studies have not demonstrated any association between silicone-gel filled breast implants and traditional auto-immune or connective tissue diseases, cancer, nor any other malignant disease. . . . EQUAM continues to believe that there is no scientific evidence that silicone allergy, silicone intoxication, atypical disease or a 'new silicone disease' exists."[123] |
| 2001 | United Kingdom | UK Independent Review Group (UK-IRG) | "There is no evidence of an association with an abnormal immune response or typical or atypical connective tissue diseases or syndromes."[124] |
| 2001 | United States | Court-appointed National Science Panel review[125] | The panel evaluated established and undifferentiated connective tissue diseases (CTD), and concluded there was no causal evidence between breast implants and these CTDs. |
| 2003 | Spain | Science and Technology Options Assessment (STOA) | The STOA report to the European Parliament Petitions Committee reported that the current scientific evidence demonstrates no solid, causal evidence linking SBI [silicone breast implants] to severe diseases, e.g. breast cancer, connective tissue diseases.[126] |
| 2009 | European Union | International Committee for Quality Assurance, Medical Technologies & Devices in Plastic Surgery panel (IQUAM) | The consensus statement of the Transatlantic Innovations conference (April 2009) indicated that additional medical studies demonstrated no association between silicone gel-filled breast implants and carcinoma, or any metabolic, immune, or allergic disorder.[127] |
| 2017 | USA | Food and Drug Administration (FDA) | Breast implants cause a rare form of cancer called Anaplastic Large Cell Lymphoma (ALCL) and is more commonly found with textured silicone and saline breast implants. “As of February 1, 2017, the FDA has received a total of 359 medical device reports of breast-implant-associated ALCL, including 9 deaths." [128] The US and European risk is 1:30,000. In Australia and New Zealand the risks are 1:1,000 to 1:10,000. |
See also
- Breast
- Breast augmentation (Augmentation mammoplasty)
- Breast enlargement supplements
- Breast reconstruction
- Breast reduction plasty
- Mammoplasty
- Mastopexy (breast lift)
- Poly Implant Prothèse
- Polypropylene breast implants
- Trans-umbilical breast augmentation (TUBA)
References
- ↑ Czerny V (1895). "Plastischer Ersatz der Brusthus durch ein Lipoma". Zentralblatt für Chirurgie. 27: 72.
- ↑ Bondurant S, Ernster V, Herdman R (eds); Committee on the Safety of Silicone Breast Implants (1999). Safety of Silicone Breast Implants. Institute of Medicine. p. 21. ISBN 0-309-06532-1.
- ↑ Anderson N (1997). "Lawsuit Science: Lessons from the Silicone Breast Implant Controversy". New York Law School Law Review. 41 (2): 401–07.
- 1 2 Stevens WG, Hirsch EM, Stoker DA, Cohen R (2006). "In vitro Deflation of Pre-filled Saline Breast Implants". Plastic and Reconstructive Surgery. 118 (2): 347–349. PMID 16874200. doi:10.1097/01.prs.0000227674.65284.80.
- ↑ "Choosing Your Breast Implants" (Web). Minneapolis Plastic Surgery, LTD. Retrieved 23 November 2016.
- ↑ Arion HG (1965). "Retromammary Prosthesis". C R Societé Française de Gynécologie. 5.
- ↑ Eisenberg TS (2009). "Silicone Gel Implants Are Back — So What?". American Journal of Cosmetic Surgery. 26: 5–7.
- ↑ Cronin TD, Gerow FJ (1963). "Augmentation Mammaplasty: A New "natural feel" Prosthesis". Excerpta Medica International Congress Series. 66: 41.
- ↑ Luu HM, Hutter JC, Bushar HF (1998). "A Physiologically based Pharmacokinetic Model for 2,4-toluenediamine Leached from Polyurethane foam-covered Breast Implants". Environ Health Perspect. 106 (7): 393–400. JSTOR 3434066. PMC 1533137
 . PMID 9637796. doi:10.2307/3434066.
. PMID 9637796. doi:10.2307/3434066. - ↑ Hester TR, Tebbetts JB, Maxwell GP (2001). "The Polyurethane-covered Mammary Prosthesis: Facts and Fiction (II): A Look Back and a "peek" Ahead". Clinical Plastic Surgery. 28 (3): 579–86. PMID 11471963.
- ↑ Brown MH, Shenker R, Silver SA (2005). "Cohesive silicone gel breast implants in aesthetic and reconstructive breast surgery". Plastic and Reconstructive Surgery. 116 (3): 768–779; discussion 779–1. PMID 16141814. doi:10.1097/01.prs.0000176259.66948.e7.
- ↑ Fruhstorfer BH, Hodgson EL, Malata CM (2004). "Early experience with an anatomical soft cohesive silicone gel prosthesis in cosmetic and reconstructive breast implant surgery". Annals of Plastic Surgery. 53 (6): 536–542. PMID 15602249. doi:10.1097/01.sap.0000134508.43550.6f.
- ↑ Hedén P, Jernbeck J, Hober M (2001). "Breast augmentation with anatomical cohesive gel implants: The world's largest current experience". Clinics in plastic surgery. 28 (3): 531–552. PMID 11471959.
- ↑ Brinton LA, Brown SL, Colton T, Burich MC, Lubin J (2000). "Characteristics of a Population of Women with Breast Implants Compared with Women Seeking other Types of Plastic Surgery". Plastic Reconstructive Surgery. 105 (3): 919–927. PMID 10724251. doi:10.1097/00006534-200003000-00014.
- ↑ Jacobsen PH, Hölmich LR, McLaughlin JK, Johansen C, Olsen JH, Kjøller K, Friis S (2004). "Mortality and suicide among Danish women with cosmetic breast implants". Arch. Intern. Med. 164 (22): 2450–5. PMID 15596635. doi:10.1001/archinte.164.22.2450.
- ↑ Young VL, Nemecek JR, Nemecek DA (1994). "The Efficacy of Breast Augmentation: Breast Size Increase, Patient Satisfaction, and Psychological Effects". Plastic Reconstructive Surgery. 94 (Dec): 958–969. PMID 7972484. doi:10.1097/00006534-199412000-00009.
- ↑ Crerand CE, Franklin ME, Sarwer DB (2006). "Body Dysmorphic Disorder and Cosmetic Surgery". Plastic Reconstructive Surgery. 118 (July): 167e–180e. PMID 17102719. doi:10.1097/01.prs.0000242500.28431.24.
- ↑ Sarwer DB, LaRossa D, Bartlett SP, Low DW, Bucky LP, Whitaker LA (2003). "Body Image Concerns of Breast Augmentation Patients". Plastic Reconstructive Surgery. 112 (July): 83–90. PMID 12832880. doi:10.1097/01.PRS.0000066005.07796.51.
- ↑ Chahraoui K, Danino A, Frachebois C, Clerc AS, Malka G (2006). "Aesthetic Surgery and Quality of Life Before and Four Months Postoperatively". Journal of the Long-Term Effects of Medical Implants. 51 (3): 207–210. PMID 16181718. doi:10.1016/j.anplas.2005.07.010.
- ↑ Cash TF, Duel LA, Perkins LL (2002). "Women's Psychosocial Outcomes of Breast Augmentation with Silicone gel-filled implants: a 2-year Prospective Study". Plastic Reconstructive Surgery. 109 (May): 2112–2121. PMID 11994621. doi:10.1097/00006534-200205000-00049.
- ↑ Figueroa-Haas CL (2007). "Effect of Breast Augmentation Mammoplasty on Self-esteem and Sexuality: A Quantitative Analysis". Plastic Surgery Nursing. 27 (Mar): 16–36. PMID 17356451. doi:10.1097/01.PSN.0000264159.30505.c9.
- ↑ "Important Information for Women About Breast Augmentation with Inamed Silicone Gel-Filled Implants" (PDF). 2006. Archived from the original (PDF) on 2007-01-03.
- ↑ Handel N, Cordray T, Gutierrez J, Jensen JA (2006). "A Long-term Study of Outcomes, Complications, and Patient Satisfaction with Breast Implants". Plastic and Reconstructive Surgery. 117 (Mar): 757–767. PMID 16525261. doi:10.1097/01.prs.0000201457.00772.1d.
- ↑ "Breast Implants Linked with Suicide in Study". Reuters. 2007-08-08.
- ↑ Manning, Anita (2007-08-06). "Breast Implants Linked to Higher Suicide Rates". USA Today. Retrieved 2010-04-26.
- ↑ Brinton LA, Lubin JH, Burich MC, Colton T, Brown SL, Hoover RN (2001). "Cancer risk at sites other than the breast following augmentation mammoplasty". Ann Epidemiol. 11 (4): 248–56. PMID 11306343. doi:10.1016/s1047-2797(00)00223-4.
- ↑ Koot VC, Peeters PH, Granath F, Grobbee DE, Nyren O (2003). "Total and cause specific mortality among Swedish women with cosmetic breast implants: prospective study". BMJ. 326 (7388): 527–8. PMC 150462
 . PMID 12623911. doi:10.1136/bmj.326.7388.527.
. PMID 12623911. doi:10.1136/bmj.326.7388.527. - ↑ Pukkala E, Kulmala I, Hovi SL, Hemminki E, Keskimäki I, Pakkanen M, Lipworth L, Boice JD, McLaughlin JK (2003). "Causes of death among Finnish women with cosmetic breast implants, 1971-2001". Ann Plast Surg. 51 (4): 339–42; discussion 343–4. PMID 14520056. doi:10.1097/01.sap.0000080407.97677.A5.
- ↑ Villeneuve PJ, Holowaty EJ, Brisson J, Xie L, Ugnat AM, Latulippe L, Mao Y (2006). "Mortality among Canadian women with cosmetic breast implants". Am. J. Epidemiol. 164 (4): 334–41. PMID 16777929. doi:10.1093/aje/kwj214.
- ↑ Brinton LA, Lubin JH, Murray MC, Colton T, Hoover RN (2006). "Mortality rates among augmentation mammoplasty patients: an update". Epidemiology. 17 (2): 162–9. PMID 16477256. doi:10.1097/01.ede.0000197056.84629.19.
- ↑ National Plastic Surgery Procedural Statistics, 2006. Arlington Heights, Illinois, American Society of Plastic Surgeons, 2007
- ↑ "Plastic Surgery Helps Self-Esteem". Psych Central.com.
- ↑ American Society of Plastic Surgeons (24 April 2014), "Five Things Physicians and Patients Should Question", Choosing Wisely: an initiative of the ABIM Foundation, American Society of Plastic Surgeons, archived from the original on 19 July 2014, retrieved 25 July 2014
- ↑ Johnson GW, Christ JE (1993). "The Endoscopic Breast augmentation: The Transumbilical Insertion of Saline-filled Breast Implants". Plastic Reconstructive Surgery. 92 (5): 801–8. PMID 8415961. doi:10.1097/00006534-199392050-00004.
- ↑ Wallach SG (2004). "Maximizing the Use of the Abdominoplasty Incision". Plastic Reconstruction Surgery. 113 (1): 411–417. PMID 14707667. doi:10.1097/01.PRS.0000091422.11191.1A.
- ↑ Graf RM, Bernardes A, Rippel R, Araujo LR, Damasio RC, Auersvald A (2003). "Subfascial Breast Implant: A New Procedure". Plastic Reconstructive Surgery. 111 (2): 904–908. PMID 12560720. doi:10.1097/01.PRS.0000041601.59651.15.
- ↑ Tebbetts JB (2004). "Does Fascia Provide Additional, Meaningful Coverage over a Breast Implant?". Plastic Reconstructive Surgery. 113 (2): 777–779. PMID 14758271. doi:10.1097/01.PRS.0000104516.13465.96.
- ↑ Tebbetts JB (2002). "A System for Breast Implant Selection Based on Patient Tissue Characteristics and Implant-soft tissue Dynamics". Plastic Reconstructive Surgery. 109 (4): 1396–1409. PMID 11964998. doi:10.1097/00006534-200204010-00030.
- ↑ Pacik PT, Nelson CE, Werner C (2008). "Pain control in augmentation mammaplasty: safety and efficacy of indwelling catheters in 644 consecutive patients". Aesthet Surg J. 28 (3): 279–84. PMID 19083538. doi:10.1016/j.asj.2008.02.001.
- ↑ Pacik PT, Nelson CE, Werner C (2008). "Pain control in augmentation mammaplasty using indwelling catheters in 687 consecutive patients: data analysis". Aesthet Surg J. 28 (6): 631–41. PMID 19083591. doi:10.1016/j.asj.2008.09.001.
- ↑ Tebbetts JB (2002). "A system for breast implant selection based on patient tissue characteristics and implant-soft tissue dynamics". Plast. Reconstr. Surg. 109 (4): 1396–409; discussion 1410–5. PMID 11964998. doi:10.1097/00006534-200204010-00030.
- ↑ Tebbetts JB, Adams WP (2005). "Five critical decisions in breast augmentation using five measurements in 5 minutes: the high five decision support process". Plast. Reconstr. Surg. 116 (7): 2005–16. PMID 16327616. doi:10.1097/01.prs.0000191163.19379.63.
- ↑ Tebbetts JB (2002). "Achieving a predictable 24-hour return to normal activities after breast augmentation: part I. Refining practices by using motion and time study principles". Plast. Reconstr. Surg. 109 (1): 273–90; discussion 291–2. PMID 11786826. doi:10.1097/00006534-200201000-00044.
- ↑ Tebbetts JB (2002). "Achieving a predictable 24-hour return to normal activities after breast augmentation: Part II. Patient preparation, refined surgical techniques, and instrumentation". Plast. Reconstr. Surg. 109 (1): 293–305; discussion 306–7. PMID 11786828. doi:10.1097/00006534-200201000-00046.
- ↑ Grippaudo FR, Renzi L, Costantino B, Longo B, Santanelli F (2013). "Late unilateral hematoma after breast reconstruction with implants: case report and literature review". Aesthetic Surgical Journal. 33 (6): 830–834. PMID 23864111. doi:10.1177/1090820X13496249.
- 1 2 "Important Information for Women About Breast Augmentation with INAMED Silicone-Filled Breast Implants" (PDF). 2006-11-03. Archived from the original (PDF) on 2007-01-03. Retrieved 2007-05-04.
- ↑ "Important Information for Augmentation Patients About Mentor MemoryGel Silicone Gel-Filled Breast Implants" (PDF). 2006-11-03. Retrieved 11 October 2014.
- ↑ "Saline-Filled Breast Implant Surgery: Making An Informed Decision (Mentor Corporation)". FDA Breast Implant Consumer Handbook - 2004. 2004-01-13. Archived from the original on 2006-11-26. Retrieved 2007-05-04.
- ↑ http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm260235.htm
- ↑ Brown SL, Middleton MS, Berg WA, Soo MS, Pennello G (2000). "Prevalence of Rupture of Silicone gel Breast Implants Revealed on MR Imaging in a Population of Women in Birmingham, Alabama". American Journal of Roentgenology. 175 (4): 1057–1064. PMID 11000165. doi:10.2214/ajr.175.4.1751057.
- ↑ Walker PS, Walls B, Murphy DK (2009). "Natrelle Saline-filled Breast Implants: a Prospective 10-year Study". Aesthetic Surgery Journal. 29 (1): 19–25. PMID 19233001. doi:10.1016/j.asj.2008.10.001.
- ↑ Hölmich LR, Vejborg IM, Conrad C, Sletting S, Høier-Madsen M, Fryzek JP, McLaughlin JK, Kjøller K, Wiik A, Friis S (2004). "Untreated Silicone Breast Implant Rupture". Plastic Reconstructive Surgery. 114 (1): 204–214. PMID 15220594. doi:10.1097/01.PRS.0000128821.87939.B5.
- ↑ Katzin WE, Centeno JA, Feng LJ, Kiley M, Mullick FG (2001). "Pathology of Lymph Nodes From Patients With Breast Implants: A Histologic and Spectroscopic Evaluation". American Journal of Surgical Pathology. 29 (4): 506–11. PMID 15767806. doi:10.1097/01.pas.0000155145.60670.e4. Archived from the original (—Scholar search) on May 24, 2009.
- ↑ "Study of Rupture of Silicone Gel-filled Breast Implants (MRI Component)". FDA Breast Implant Consumer Handbook - 2004. 2000-05-22. Retrieved 2007-05-04.
- 1 2 3 "Local Complications". FDA Breast Implant Consumer Handbook - 2004. 2004-06-08. Archived from the original on 2007-05-13. Retrieved 2007-05-04.
- ↑ MRI of a ruptured silicone breast implant 2013-04-05
- ↑ Hölmich LR, Friis S, Fryzek JP, Vejborg IM, Conrad C, Sletting S, Kjøller K, McLaughlin JK, Olsen JH (2003). "Incidence of Silicone Breast Implant Rupture". Arch Surg. 138 (7): 801–806. PMID 12860765. doi:10.1001/archsurg.138.7.801.
- ↑ Hedén P, Nava MB, van Tetering JP, Magalon G, Fourie le R, Brenner RJ, Lindsey LE, Murphy DK, Walker PS (2006). "Prevalence of Rupture in Inamed Silicone Breast Implants". Plastic Reconstructive Surgery. 118 (2): 303–308. PMID 16874191. doi:10.1097/01.prs.0000233471.58039.30.
- ↑ "FDA summary of clinical issues (MS Word document)".
- ↑ Cunningham B, McCue J (2009). "Safety and effectiveness of Mentor's MemoryGel implants at 6 years". Plastic Reconstructive Surgery. 33 (3): 440–444. PMID 19437068. doi:10.1007/s00266-009-9364-6.
- ↑ Hedén P, Boné B, Murphy DK, Slicton A, Walker PS (2006). "Style 410 Cohesive Silicone Breast Implants: Safety and Effectiveness at 5 to 9 years after Implantation". Plastic Reconstructive Surgery. 118 (6): 1281–1287. PMID 17051096. doi:10.1097/01.prs.0000239457.17721.5d.
- ↑ Hölmich LR, Fryzek JP, Kjøller K, Breiting VB, Jørgensen A, Krag C, McLaughlin JK (2005). "The Diagnosis of Silicone Breast implant Rupture: Clinical Findings Compared with Findings at Magnetic Resonance Imaging". Annals of Plastic Surgery. 54 (6): 583–589. PMID 15900139. doi:10.1097/01.sap.0000164470.76432.4f.
- ↑ "Expert Advisory Panel on Breast Implants: Record of Proceedings". HealthCanada. 2005-09-29. Archived from the original on 2007-11-07. Retrieved 2007-05-04.
- ↑ Song JW, Kim HM, Bellfi LT, Chung KC (2011). "The Effect of Study design Biases on the Diagnostic Accuracy of Magnetic Resonance Imaging for Detecting Silicone Breast Implant Ruptures: a Meta-analysis". Plastic and Reconstructive Surgery. 127 (3): 1029–1044. PMC 3080104
 . PMID 21364405. doi:10.1097/PRS.0b013e3182043630.
. PMID 21364405. doi:10.1097/PRS.0b013e3182043630. - ↑ AFP (18 September 2011). "Breast implants safe, but not for life: US experts". The Independent.
- ↑ Barnsley GP, Sigurdson LJ, Barnsley SE (2006). "Textured surface Breast Implants in the Prevention of Capsular Contracture among Breast Augmentation Patients: a Meta-analysis of Randomized Controlled Trials". Plastic Reconstructive Surgery. 117 (7): 2182–2190. PMID 16772915. doi:10.1097/01.prs.0000218184.47372.d5.
- ↑ Wong CH, Samuel M, Tan BK, Song C (2006). "Capsular Contracture in Subglandular Breast Augmentation with Textured versus Smooth Breast Implants: a Systematic Review". Plastic Reconstructive Surgery. 118 (5): 1224–1236. PMID 17016195. doi:10.1097/01.prs.0000237013.50283.d2.
- ↑ Handel N, Gutierrez J (May 2006). "Long-term safety and efficacy of polyurethane foam-covered breast implants". Journal of Aesthetic Surgery. 26 (3): 265–274. PMID 19338905. doi:10.1016/j.asj.2006.04.001.
- ↑ Mladick RA (1993). ""No-touch" submuscular saline breast augmentation technique". Journal of Aesthetic Surgery. 17 (3): 183–192. PMID 8213311. doi:10.1007/BF00636260.
- ↑ Adams WP, Rios JL, Smith SJ (2006). "Enhancing Patient Outcomes in Aesthetic and Reconstructive Breast Surgery using Triple Antibiotic Breast Irrigation: Six-year Prospective Clinical Study". Plastic Reconstructive Surgery. 117 (1): 30–6. PMID 16404244. doi:10.1097/01.prs.0000185671.51993.7e.
- ↑ Planas J, Cervelli V, Planas G (2001). "Five-year experience on ultrasonic treatment of breast contractures". Aesthetic Plastic Surgery. 25 (2): 89–93. PMID 11349308. doi:10.1007/s002660010102.
- ↑ Schlesinger SL, Ellenbogen R, Desvigne MN, Svehlak S, Heck R (2002). "Zafirlukast (Accolate): A new treatment for capsular contracture". Aesthetic Plast Surg. 22 (4): 329–36. PMID 19331987. doi:10.1067/maj.2002.126753.
- ↑ Scuderi N, Mazzocchi M, Fioramonti P, Bistoni G (2006). "The effects of zafirlukast on capsular contracture: preliminary report". Aesthetic Plast Surg. 30 (5): 513–520. PMID 16977359. doi:10.1007/s00266-006-0038-3.
- ↑ Silver H (1982). "Reduction of capsular contracture with two-stage augmentation mammaplasty and pulsed electromagnetic energy (Diapulse therapy)". Plastic Reconstructive Surgery. 69 (5): 802–805. PMID 7071225. doi:10.1097/00006534-198205000-00013.
- ↑ Tebbetts JB (October 2006). "Out Points Criteria for Breast Implant Removal without Replacement and Criteria to Minimize Reoperations following Breast Augmentation". Plastic Reconstructive Surgery. 114 (5): 1258–1262. PMID 15457046. doi:10.1097/01.prs.0000136802.91357.cf.
- ↑ Tebbetts JB (December 2006). "Achieving a Zero Percent Reoperation Rate at 3 years in a 50-consecutive-case Augmentation Mammaplasty Premarket Approval Study". Plastic Reconstructive Surgery. 118 (6): 1453–7. PMID 17051118. doi:10.1097/01.prs.0000239602.99867.07.
- ↑ Brawer, AE. "Chronology of systemic disease development in 300 symptomatic recipients of silicone gel-filled breast implants." J. Clean Technology, Environmental Toxicology, and Occupational Med 1996:5, #3, 223-233.
- ↑ Borenstein, David. "Siliconosis: A spectrum of illness." Seminars in Arthritis and Rheumatism 1994: 24, #1 1-7.
- ↑ Cichon, MJ; Farrel, DL; Ganio, SH; Sadler, GM. "A case series survey of silicone breast implant patients." Journal of Chronic Fatigue System 1999: 5, #3-4.
- ↑ Maijers, MC; Blok de, CJM; Niessen, FB; Veldt van der, Ritt, MJPF; Winters, HAH; Kramer, MHH; Nanayakkara, PWB. "Women with silicone breast implants and unexplained systemic symptoms: a descriptive cohort study." Netherlands The Journal of Medicine. 2013: 71, #10.
- ↑ Patten, BM; Shoaib, BO; Calkins, DS. "Adjuvant breast disease: an evaluation of 100 symptomatic women with breast implants or silicone fluid injections." The Keio Journal of Medicine 1994; #2 79-87.
- ↑ Breast Implant Illness
- ↑ Maharaj SVM. Platinum concentration in silicone breast implant material and capsular tissue by IVP-MS. Anal Bioanal Chem (2004) 380: 84-89
- ↑ Lykissa, ED; Maharaj, SVM. Total Platinum Concentration and Platinum Oxidation States in Body Fluids, Tissue, and Explants from Women Exposed to Silicon and Saline Breast Implants by IC-ICP-MS. Analytical Chemistry (published on-ilne April 1, 2006)
- ↑ Maharaj, SVM; Lykissa, ED. Total platinum in urine of women exposed to silicone breast implants and in their children conceived after implantation by ICP-MS. Abstract presented to the American Chemical Society Meeting 2005.
- ↑ <Breast Implant Illness>
- ↑ https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm547622.htm
- ↑ FDA - Medical Device Reports of Breast Implant Associated Anaplastic Large Cell Lymphoma.
- 1 2 Clemens, Mark. "Breast Implant Associated Anaplastic Large Cell Lymphoma (BIA-ALCL)" (2017).
- ↑ Clemens, Mark W.; Horwitz, Steven M. (2017-03-01). "NCCN Consensus Guidelines for the Diagnosis and Management of Breast Implant-Associated Anaplastic Large Cell Lymphoma". Aesthetic Surgery Journal. 37 (3): 285–289. ISSN 1527-330X. PMID 28184418. doi:10.1093/asj/sjw259.
- ↑ "Breast Implant Associated ALCL: PROFILE Project | The Plastic Surgery Foundation". www.thepsf.org. Retrieved 2017-04-25.
- ↑ Berlin CM (1994). "Silicone Breast Implants and Breast-feeding". Pediatrics. 94 (4 Pt 1): 546–549. PMID 7936870.
- ↑ Berlin, Cheston M., Jr. Silicone Breast Implants and Breastfeeding, Hershey Medical Center, Hershey, Pennsylvania; from Breastfeeding Abstracts. February 1996, Volume 15, Number 3, pp. 17–18.
- ↑ Breastfeeding after Breast Surgery, La Leche League, contains references.
- ↑ Breastfeeding and Breast Implants, Selected Bibliography April 2003, LLLI Center for Breastfeeding Information
- 1 2 Inorganic Milk: Can Kendra Wilkinson breast-feed her baby even though she has implants?, Christopher Beam, Slate.com, 11 December 2009
- ↑ Handel N, Silverstein MJ, Gamagami P, Jensen JA, Collins A (1992). "Factors Affecting Mammographic Visualization of the Breast after Augmentation Mammaplasty". JAMA. 268 (14): 1913–1917. PMID 1404718. doi:10.1001/jama.268.14.1913.
- ↑ Clark CP, Peters GN, O'Brien KM (1993). "Cancer in the Augmented Breast: Diagnosis and Prognosis". Cancer. 72 (7): 2170–4. PMID 8374874. doi:10.1002/1097-0142(19931001)72:7<2170::AID-CNCR2820720717>3.0.CO;2-1.
- ↑ Skinner KA, Silberman H, Dougherty W, Gamagami P, Waisman J, Sposto R, Silverstein MJ (2001). "Breast cancer after augmentation mammoplasty". Ann Surg Oncol. 8 (2): 138–44. PMID 11258778. doi:10.1007/s10434-001-0138-x.
- 1 2 Le GM, O'Malley CD, Glaser SL, Lynch CF, Stanford JL, Keegan TH, West DW (2005). "Breast implants following mastectomy in women with early-stage breast cancer: prevalence and impact on survival". Breast Cancer Res. 7 (2): R184–93. PMC 1064128
 . PMID 15743498. doi:10.1186/bcr974.
. PMID 15743498. doi:10.1186/bcr974. - ↑ Handel N, Silverstein MJ (2006). "Breast cancer diagnosis and prognosis in augmented women". Plastic Reconstructive Surgery. 118 (3): 587–93. PMID 16932162. doi:10.1097/01.prs.0000233038.47009.04.
- ↑ Cunningham B (2006). "Breast cancer diagnosis and prognosis in augmented women- Discussion". Plastic Reconstructive Surgery. 118 (3): 594–595. doi:10.1097/01.prs.0000233047.87102.8e.
- ↑ Schwartz GF, Veronesi U, Clough KB, Dixon JM, Fentiman IS, Heywang-Köbrunner SH, Holland R, Hughes KS, Mansel RE, Margolese R, Mendelson EB, Olivotto IA, Palazzo JP, Solin LJ (2006). "Consensus Conference on Breast Conservation". JACAS. 203 (2): 198–207. PMID 16864033. doi:10.1016/j.jamcollsurg.2006.04.009.
- ↑ Breast Cancer Detection and Survival among Women with Cosmetic Breast Implants: Systematic Review and Meta-analysis of Observational Studies. Lavigne, Holowaty, Pan, Villeneuve, Johnson, Fergusson, Morrison, and Brisson. BMJ (2013): n. pag. Web. <http://www.bmj.com/content/346/bmj.f2399>.
- ↑ Hwang ES; et al. (April 2013). "Survival after Lumpectomy and Mastectomy for Early stage Invasive Breast Cancer: The Effect of Age and Hormone receptor Status". Cancer. 119: 1402–1411. doi:10.1002/cncr.27795.
- 1 2 3 4 5 6 7 8 FDA Breast Implant Consumer Handbook - 2004 Archived 2008-09-17 at the Wayback Machine.
- ↑ "Safety of Silicone Breast Implants - The National Academies Press". nap.edu.
- ↑ Martha Grigg, Stuart Bondurant, Virginia L. Ernster, and Roger Herdman, Editors. "Information for Women about the Safety of Silicone Breast Implants - The National Academies Press". nap.edu.
- ↑ FDA study Archived January 13, 2008, at the Wayback Machine.
- ↑ FDA approval
- ↑ FDA approval
- ↑ "FDA Approves Silicone Gel-Filled Breast Implants". FDA. Retrieved 2008-07-01.
- ↑
- ↑
- ↑ Breiting VB, Hölmich LR, Brandt B, Fryzek JP, Wolthers MS, Kjøller K, McLaughlin JK, Wiik A, Friis S (2004). "Long-term Health Status of Danish Women with Silicone Breast Implants". Plastic and Reconstructive Surgery. 114 (1): 217–226. PMID 15220596. doi:10.1097/01.PRS.0000128823.77637.8A.
- ↑ Brinton LA, Lubin JH, Murray MC, Colton T, Hoover RN (2006). "Mortality Rates Among Augmentation Mammoplasty Patients: An Update". Epidemiology. 17 (2): 162–9. PMID 16477256. doi:10.1097/01.ede.0000197056.84629.19.
- ↑ Villeneuve PJ, Holowaty EJ, Brisson J, Xie L, Ugnat AM, Latulippe L, Mao Y (June 2006). "Mortality Among Canadian Women with Cosmetic Breast Implants". American Journal of Epidemiology. 164 (4): 334–341. PMID 16777929. doi:10.1093/aje/kwj214.
- ↑ Brinton LA, Malone KE, Coates RJ, Schoenberg JB, Swanson CA, Daling JR, Stanford JL (1996). "Breast Enlargement and Reduction: Results from a Breast Cancer Case-control Study". Plastic Reconstructive Surgery. 97 (2): 269–275. PMID 8559808. doi:10.1097/00006534-199602000-00001.
- ↑ Benadiba, Laurent (2004). "Histoire des protheses mammaires" (in French). Archived from the original on 29 January 2015. Retrieved 12 October 2015.
- ↑ http://www.tga.gov.au/docs/pdf/breasti4.pdf
- ↑ "German Society for Senology, Declaration of Consensus for the Security of Silicone Breast Implants-24 September 1998". 1998.
- ↑ Janowsky EC, Kupper LL, Hulka BS (2000). "Meta-analyses of the Relation between Silicone Breast Implants and the Risk of Connective-tissue Diseases". New England Journal of Medicine. 342 (11): 781–790. PMID 10717013. doi:10.1056/NEJM200003163421105.
- ↑ Archived December 27, 2005, at the Wayback Machine.
- ↑ Archived June 23, 2006, at the Wayback Machine.
- ↑ Tugwell P, Wells G, Peterson J, Welch V, Page J, Davison C, McGowan J, Ramroth D, Shea B (2001). "Do silicone Breast Implants Cause Rheumatologic Disorders? A Systematic Review for a Court-appointed National Science Panel". Arthritis Rheum. 44 (11): 2477–84. PMID 11710703. doi:10.1002/1529-0131(200111)44:11<2477::AID-ART427>3.0.CO;2-Q.
- ↑ https://web.archive.org/web/20030829114951/http://www.eucomed.be/docs/STOA-SILICONE%20BREAST%20IMPLANT%20Study%20update-30May03.pdf
- ↑ Neuhann-Lorenz C, Fedeles J, Eisenman-Klein M, Kinney B, Cunningham BL (2001). "Eighth IQUAM Consensus Position Statement: Transatlantic Innovations, April 2009". Plastic and Reconstructive Surgery. 127 (3): 1368–75. PMID 21364439. doi:10.1097/PRS.0b013e318206312e.
- ↑ FDA - Medical Device Reports of Breast Implant Associated Anaplastic Large Cell Lymphoma.
External links
| Wikimedia Commons has media related to Breast implants. |
- Basics of implant based breast reconstruction (E-medicine)
- Institute of Medicine (IOM) Report on Silicone Implants
- U.S. Food and Drug Administration (FDA)—breast implant page
- U.K. Medicines & Health Care Products Regulatory Agency (MHRA)—breast implant page
- Australia's Department of Health & Aging Therapeutic Goods Administration breast implant page
- Breast Implant Illness
- What you need to know about breast implants by the National Center for Health Research