Fecal incontinence
| Fecal incontinence | |
|---|---|
| Synonyms | Faecal incontinence, bowel incontinence, anal incontinence, accidental bowel leakage |
 | |
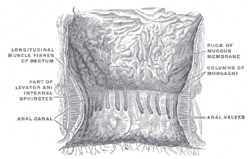
| Diagram showing normal anatomy of anal canal and rectum. | |
| Classification and external resources | |
| ICD-10 | R15 |
| ICD-9-CM | 787.6 |
| DiseasesDB | 6763 |
| MedlinePlus | 003135 |
| eMedicine | article/268674 |
| MeSH | D005242 |
Fecal incontinence (FI), or in some forms encopresis, is a lack of control over defecation, leading to involuntary loss of bowel contents—including flatus (gas), liquid stool elements and mucus, or solid feces. FI is a sign or a symptom, not a diagnosis. Incontinence can result from different causes and might occur with either constipation or diarrhea. Continence is maintained by several inter-related factors, including the anal sampling mechanism, and usually there is more than one deficiency of these mechanisms for incontinence to develop. The most common causes are thought to be immediate or delayed damage from childbirth, complications from prior anorectal surgery (especially involving the anal sphincters or hemorrhoidal vascular cushions) and altered bowel habits (e.g., caused by irritable bowel syndrome, Crohn's disease, ulcerative colitis, food intolerance, or constipation with overflow incontinence).[1] An estimated 2.2% of community dwelling adults are affected.[2]
Fecal incontinence has three main consequences: local reactions of the perianal skin and urinary tract, including maceration (softening and whitening of skin due to continuous moisture), urinary tract infections, or decubitus ulcers (pressure sores);[1] a financial expense for individuals (due to cost of medication and incontinence products, and loss of productivity), employers (days off), and medical insurers and society generally (health care costs, unemployment); and an associated decrease in quality of life.[3] There is often reduced self-esteem, shame, humiliation, depression, a need to organize life around easy access to bathroom and avoidance of enjoyable activities. FI is an example of a stigmatized medical condition, which creates barriers to successful management. People may be too embarrassed to seek medical help, and attempt to self-manage the symptom in secrecy from others.
FI is one of the most psychologically and socially debilitating conditions in an otherwise healthy individual, but it is generally treatable.[2] Management may be achieved through an individualized mix of dietary, pharmacologic, and surgical measures. Health care professionals are often poorly informed about treatment options,[2] and may fail to recognize the effect of FI.[3]
Signs and symptoms
FI affects virtually all aspects of peoples' lives, greatly diminishing physical and mental health, and affect personal, social and professional life. Emotional effects may include stress, fearfulness, anxiety, exhaustion, fear of public humiliation, feeling dirty, poor body-image, reduced desire for sex, anger, humiliation, depression, isolation, secrecy, frustration and embarrassment. Some people may need to be in control of life outside of FI as means of compensation. The physical symptoms such as skin soreness, pain and odor may also affect quality of life. Physical activity such as shopping or exercise is often affected. Travel may be affected, requiring careful planning. Working is also affected for most. Relationships, social activities and self-image likewise often suffer.[4] Symptoms may worsen over time.[1]
Causes
FI is a sign or a symptom, not a diagnosis,[4] and represents an extensive list of causes. Usually, it is the result of a complex interplay of several coexisting factors, many of which may be simple to correct.[4] Up to 80% of people may have more than one abnormality that is contributing.[5] Deficits of individual functional components of the continence mechanism can be partially compensated for a certain period of time, until the compensating components themselves fail. For example, obstetric injury may precede onset by decades, but postmenopausal changes in the tissue strength reduce in turn the competence of the compensatory mechanisms.[1][6] The most common factors in the development are thought to be obstetric injury and after effects of anorectal surgery, especially those involving the anal sphincters and hemorrhoidal vascular cushions.[1] The majority of incontinent persons over the age of 18 fall into one of several groups. These are: those with structural anorectal abnormalities (sphincter trauma, sphincter degeneration, perianal fistula, rectal prolapse), neurological disorders (multiple sclerosis, spinal cord injury, spina bifida, stroke, etc.), constipation/fecal loading (presence of a large amount of feces in the rectum with stool of any consistency), cognitive and/or behavioral dysfunction (dementia, learning disabilities), diarrhea, inflammatory bowel diseases (e.g. ulcerative colitis, Crohn's disease), irritable bowel syndrome, disability related (people who are frail, acutely unwell, or have chronic/acute disabilities), and those cases which are idiopathic (of unknown cause).[4][7] Diabetes mellitus is also known to be a cause, but the mechanism of this relationship is not well understood.[8]
Congenital
Anorectal anomalies and spinal cord defects may be a cause in children. These are usually picked up and operated upon during early life, but continence is often imperfect thereafter.[2]
Anal canal
The functioning of the anal canal can be damaged, traumatically or atraumatically. The resting tone of the anal canal is not the only factor which is important, both the length of the high pressure zone and its radial translation of force are required for continence. This means that even with normal anal canal pressure, focal defects such as the keyhole deformity can be the cause of substantial symptoms. External anal sphincter (EAS) dysfunction is associated with impaired voluntary control, whereas internal anal sphincter (IAS) dysfunction is associated with impaired fine tuning of fecal control.[1] Lesions which mechanically interfere with, or prevent the complete closure of the anal canal can cause a liquid stool or mucous rectal discharge. Such lesions include piles (inflamed hemorrhoids), anal fissures, anal cancer or fistulae. Obstetric injury may tear the anal sphincters, and some of these injuries may be occult (undetected). The risk of injury is greatest when labor has been especially difficult or prolonged, when forceps are used, with higher birth weights or when an midline episiotomy is performed. Only when there is post operative investigation of FI such as endoanal ultrasound is the injury discovered.[2] FI is a much under-reported complication of surgery. The IAS is easily damaged with an anal retractor (especially the Park's anal retractor), leading to reduced resting pressure postoperatively. Since the hemorrhoidal vascular cushions contribute 15% of the resting anal tone, surgeries involving these structures may affect continence status.[2] Partial internal sphincterotomy, fistulotomy, anal stretch (Lord's operation), hemorrhoidectomy or transanal advancement flaps may all lead to FI post operatively, with soiling being far more common than solid FI. The "keyhole deformity" refers to scarring within the anal canal and is another cause of mucus leakage and minor incontinence. This defect is also described as a groove in the anal canal wall, and may occur after posterior midline fissurectomy or fistulotomy, or with lateral IAS defects. Rare causes of traumatic injury to the anal sphincters include military or traffic accidents complicated by pelvic fractures, spine injuries or perineal lacerations, insertion of foreign bodies in the rectum, and sexual abuse.[2] Nontraumatic conditions causing anal sphincter weakness include scleroderma, damage to the pudendal nerves and IAS degeneration of unknown cause.[3] Radiation induced FI may involve the anal canal as well as the rectum, when proctitis, anal fistula formation and diminished function of internal and external sphincter occur.[2] Irradiation may occur during radiotherapy, e.g. for prostate cancer.
Pelvic floor
Many people with FI have a generalized weakness of the pelvic floor, especially puborectalis.[3] A weakened puborectalis leads to widening of the anorectal angle, and impaired barrier to stool in the rectum entering the anal canal, and this is associated with incontinence to solids. Abnormal descent of the pelvic floor can also be a sign of pelvic floor weakness. Abnormal descent manifests as descending perineum syndrome (>4 cm perineal descent).[3] This syndrome initially gives constipation, and later FI. The pelvic floor is innervated by the pudendal nerve and the S3 and S4 branches of the pelvic plexus. With recurrent straining, e.g. during difficult labour or long term constipation, then stretch injury can damage the nerves supplying levator ani. The pudendal nerve is especially vulnerable to irreversible damage, (stretch induced pudendal neuropathy) which can occur with a 12% stretch.[2] If the pelvic floor muscles lose their innervation, they cease to contract and their muscle fibres are in time replaced by fibrous tissue, which is associated with pelvic floor weakness and incontinence. Increased pudendal nerve terminal motor latency may indicate pelvic floor weakness. The various types of pelvic organ prolapse (e.g. external rectal prolapse, mucosal prolapse and internal rectal intussusception & solitary rectal ulcer syndrome) may also cause coexisting obstructed defecation.
Rectum
The rectum needs to be of a sufficient volume to store stool until defecation. The rectal walls need to be "compliant" i.e. able to distend to an extent to accommodate stool. Rectal sensation is required to detect the presence, nature and amount of rectal contents. The rectum must also be able to evacuate its contents fully. There must also be efficient co-ordination of rectal sensation and relaxation of the anal canal.[9] Rectal storage capacity (i.e. rectal volume + rectal compliance) may be affected in the following ways. Surgery involving the rectum (e.g. lower anterior resection, often performed for colorectal cancer), radiotherapy directed at the rectum, and inflammatory bowel disease can cause scarring, which may result in the walls of the rectum becoming stiff and inelastic, reducing compliance. Reduced rectal storage capacity may lead to urge incontinence, where there is an urgent need to defecate as soon as stool enters the rectum, where normally stool would be stored until there was enough to distend the rectal walls and initiate the defecation cycle. Tumors and strictures also may impair reservoir function. Conversely, increased rectal volume (megarectum), may cause fecal loading and overflow FI. Reduced rectal sensation may be a contributory factor. If the sensory nerves are damaged, detection of stool in the rectum is dulled or absent, and the person will not feel the need to defecate until too late. Rectal hyposensitivity may manifest as constipation, FI, or both. Rectal hyposensitivty was reported to be present in 10% of people with FI.[10] Pudendal neuropathy is one cause of rectal hyposensitivity, and may lead to fecal loading/impaction, megarectum and overflow FI.[11] Normal evacuation of rectal contents is 90-100%.[2] If there is incomplete evacuation during defecation, residual stool will be left in the rectum and threaten continence once defecation is finished. This is a feature of people with soiling secondary to obstructed defecation.[12] Obstructed defecation is often due to anismus (paradoxical contraction or relaxation failure of the puborectalis).[2]:38 Whilst anismus is largely a functional disorder, organic pathologic lesions may mechanically interfere with rectal evacuation. Other causes of incomplete evacuation include non-emptying defects like a rectocele. Straining to defecate pushes stool into the rectocele, which acts like a diverticulum and causes stool sequestration. Once the voluntary attempt to defecate, albeit dysfunctional, is finished, the voluntary muscles relax, and residual rectal contents are then able to descend into the anal canal and cause leaking.[2]:37
| Drug/mechanism of action | Common examples |
|---|---|
| Drugs altering sphincter tone |
Nitrates, calcium channel antagonists, beta-adrenoceptor antagonists (beta-blockers), sildenafil, selective serotonin reuptake inhibitors |
| Broad spectrum antibiotics | |
| Topical drugs applied to anus (reducing pressure) |
Glyceryl trinitrate ointment, diltiazem gel, bethanechol cream, botulinum toxin A injection |
| Drugs causing profuse diarrhea |
Laxatives, metformin, orlistat, selective serotonin reuptake inhibitors, magnesium-containing antacids, digoxin |
| Constipating drugs |
Loperamide, opioids, tricyclic antidepressants, aluminium-containing antacids, codeine |
| Tranquilisers/hypnotics (reducing alertness) |
Benzodiazepines, tricyclic antidepressants, selective serotonin reuptake inhibitors, anti-psychotics |
Central nervous system
Continence requires conscious and subconscious networking of information from and to the anorectum. Defects/brain damage may affect the central nervous system focally (e.g. stroke, tumor e.g. spinal cord lesions, trauma, multiple sclerosis) or diffusely (e.g. dementia, multiple sclerosis, infection, Parkinson's disease or drug-induced).[1][14] FI (and urinary incontinence) may also occur during epileptic seizures.[15] Dural ectasia is an example of a spinal cord lesion that may affect continence.[16]
Diarrhea
Liquid stool is more difficult to control than formed, solid stool. Hence, FI can be exacerbated by diarrhea.[4] Some consider diarrhea to be the most common aggravating factor.[2] Where diarrhea is caused by temporary problems such as mild infections or food reactions, incontinence tends to be short lived. Chronic conditions, such as irritable bowel syndrome or Crohn's disease, can cause severe diarrhea lasting for weeks or months. Diseases, drugs, and indigestible dietary fats that interfere with the intestineal absorption may cause steatorrhea (oily rectal discharge & fatty diarrhea) and degrees of FI. Respective examples include cystic fibrosis, orlistat, and olestra. Postcholecystectomy diarrhea is diarrhea that occurs following gall bladder removal, due to excess bile acid. Orlistat is an anti-obesity (weight loss) drug that blocks the absorption of fats. This may give side effects of FI, diarrhea and steatorrhea.[17]
Overflow incontinence
This may occur when there is a large mass of feces in the rectum (fecal loading), which may become hardened (fecal impaction). Liquid stool elements are able to pass around the obstruction, leading to incontinence. Megarectum (enlarged rectal volume) and rectal hyposensitivity are associated with overflow incontinence. Hospitalized patients and care home residents may develop FI via this mechanism,[4] possibly a result of lack of mobility, reduced alertness, constipating effect of medication and/or dehydration.
Pathophysiology
_Stylized_depiction_of_action_of_puborectalis_sling.png)
The mechanisms and factors contributing to normal continence are multiple and inter-related. The puborectalis sling, forming the anorectal angle (see diagram), is responsible for gross continence of solid stool.[3] The IAS is an involuntary muscle, contributing about 55% of the resting anal pressure. Together with the hemorrhoidal vascular cushions, the IAS maintains continence of flatus and liquid during rest. The EAS is a voluntary muscle, doubling the pressure in the anal canal during contraction, which is possible for a short time. The rectoanal inhibitory reflex (RAIR) is an involuntary IAS relaxation in response to rectal distension, allowing some rectal contents to descend into the anal canal where it is brought into contact with specialized sensory mucosa to detect consistency. The rectoanal excitatory reflex (RAER) is an initial, semi-voluntary contraction of the EAS and puborectalis which in turn prevents incontinence following the RAIR. Other factors include the specialized anti-peristaltic function of the last part of the sigmoid colon, which keeps the rectum empty most of the time, sensation in the lining of the rectum and the anal canal to detect when there is stool present, its consistency and quantity, and the presence of normal rectoanal reflexes and defecation cycle which completely evacuates stool from the rectum and anal canal. Problems affecting any of these mechanisms and factors may be involved in the cause.[2]
Diagnostic approach
Identification of the exact causes usually begins with a thorough medical history, including detailed questioning about symptoms, bowel habits, diet, medication and other medical problems. Digital rectal examination is performed to assesses resting pressure and voluntary contraction (maximum squeeze) of the sphincter complex and puborectalis. Anal sphincter defects, rectal prolapse, and abnormal perineal descent may be detected.[3] Anorectal physiology tests assess the functioning of the anorectal anatomy. Anorectal manometry records the pressure exerted by the anal sphincters and puborectalis during rest and during contraction. The procedure is also able to assess sensitivity of the anal canal and rectum. Anal electromyography tests for nerve damage, which is often associated with obstetric injury. Pudendal nerve terminal motor latency tests for damage to the pudendal motor nerves. Proctography, also known as defecography, shows how much stool the rectum can hold, how well the rectum holds it, and how well the rectum can evacuate the stool. It will also highlight defects in the structure of the rectum such as internal rectal intussusception. Dynamic pelvic MRI, also called MRI defecography is an alternative which is better for some problems but not as good for other problems.[18] Proctosigmoidoscopy involves the insertion of an endoscope (a long, thin, flexible tube with a camera) into the anal canal, rectum and sigmoid colon. The procedure allows for visualization of the interior of the gut, and may detect signs of disease or other problems that could be a cause, such as inflammation, tumors, or scar tissue. Endoanal ultrasound, which some consider to be the gold standard for detection of anal canal lesions,[19] evaluates the structure of the anal sphincters, and may detect occult sphincter tears that otherwise would go unseen.
Functional FI is common.[20] The Rome process published diagnostic criteria for functional FI, which they defined as "recurrent uncontrolled passage of fecal material in an individual with a developmental age of at least 4 years". The diagnostic criteria are, one or more of the following factors present for the last 3 months: abnormal functioning of normally innervated and structurally intact muscles, minor abnormalities of sphincter structure/innervation (nerve supply), normal or disordered bowel habits, (i.e., fecal retention or diarrhea), and psychological causes. Furthermore, exclusion criteria are given. These are factors which all must be excluded for a diagnosis of functional FI, and are abnormal innervation caused by lesion(s) within the brain (e.g., dementia), spinal cord (at or below T12), or sacral nerve roots, or mixed lesions (e.g., multiple sclerosis), or as part of a generalized peripheral or autonomic neuropathy (e.g., due to diabetes), anal sphincter abnormalities associated with a multisystem disease (e.g., scleroderma), and structural or neurogenic abnormalities that are the major cause.[21]
Definition
There is no globally accepted definition,[1] but fecal incontinence is generally defined as the recurrent inability to voluntarily control the passage of bowel contents through the anal canal and expel it at a socially acceptable location and time, occurring in individuals over the age of four.[1][2][3][4][6] "Social continence" has been given various precise definitions for the purposes of research, however generally it refers to symptoms being controlled to an extent that is acceptable to the individual in question, with no significant effect on their life. There is no consensus about the best way to classify FI,[4] and several methods are used.
Symptoms can be directly or indirectly related to the loss of bowel control. The direct (primary) symptom is a lack of control over bowel contents which tends to worsen without treatment. Indirect (secondary) symptoms, which are the result of leakage, include pruritus ani (an intense itching sensation from the anus), perianal dermatitis (irritation and inflammation of the skin around the anus), and urinary tract infections.[1] Due to embarrassment, people may only mention secondary symptoms rather than acknowledge incontinence. Any major underlying cause will produce additional signs and symptoms, such as protrusion of mucosa in external rectal prolapse. Symptoms of fecal leakage (FL) are similar, and may occur after defecation. There may be loss of small amounts of brown fluid and staining of the underwear.[2]
Types
FI can be divided into those people who experience a defecation urge before leakage (urge incontinence), and those who experience no sensation before leakage (passive incontinence or soiling).[4] Urge incontinence is characterized by a sudden need to defecate, with little time to reach a toilet. Urge and passive FI may be associated with weakness of the external anal sphincter (EAS) and internal anal sphincter (IAS) respectively. Urgency may also be associated with reduced rectal volume, reduced ability of the rectal walls to distend and accommodate stool, and increased rectal sensitivity.[3]
There is a continuous spectrum of different clinical presentations from incontinence of flatus (gas), through incontinence of mucus or liquid stool, to solids. The term anal incontinence often is used to describe flatus incontinence,[4] however it is also used as a synonym for FI generally. It may occur together with incontinence of liquids or solids, or it may present in isolation. Flatus incontinence may be the first sign of FI.[2] Once continence to flatus is lost, it is rarely restored.[4] Anal incontinence may be equally disabling as the other types.[22] Fecal leakage, fecal soiling and fecal seepage are minor degrees of FI, and describe incontinence of liquid stool, mucus, or very small amounts of solid stool. They cover a spectrum of increasing symptom severity (staining, soilage, seepage and accidents).[1] Rarely, minor FI in adults may be described as encopresis. Fecal leakage is a related topic to rectal discharge, but this term does not necessarily imply any degree of incontinence. Discharge generally refers to conditions where there is pus or increased mucus production, or anatomical lesions that prevent the anal canal from closing fully, whereas fecal leakage generally concerns disorders of IAS function and functional evacuation disorders which cause a solid fecal mass to be retained in the rectum. Solid stool incontinence may be called complete (or major) incontinence, and anything less as partial (or minor) incontinence (i.e. incontinence of flatus (gas), liquid stool and/or mucus).[2]
In children over the age of four who have been toilet trained, a similar condition is generally termed encopresis (or soiling), which refers to the voluntary or involuntary loss of (usually soft or semi-liquid) stool.[23] The term pseudoincontinence is used when there is FI in children who have anatomical defects (e.g. enlarged sigmoid colon or anal stenosis).[2] Encopresis is a term that is usually applied when there are no such anatomical defects present. The ICD-10 classifies nonorganic encopresis under "behavioural and emotional disorders with onset usually occurring in childhood and adolescence" and organic causes of encopresis along with FI.[24] FI can also be classified according to gender, since the cause in females may be different from males, for example it may develop following radical prostatectomy in males,[25] whereas females may develop FI as an immediate or delayed consequence of damage whilst giving birth. Pelvic anatomy is also different according to gender, with a wider pelvic outlet in females.
Clinical measurement
Several severity scales exist. The Cleveland Clinic (Wexner) fecal incontinence score takes into account five parameters that are scored on a scale from zero (absent) to four (daily) frequency of incontinence to gas, liquid, solid, of need to wear pad, and of lifestyle changes.[1] The Park's incontinence score uses four categories:
- 1 - those continent for solid and liquid stool and also for flatus.
- 2 - those continent for solid and liquid stool but incontinent for flatus (with or without urgency).
- 3 - those continent for solid stool but incontinent for liquid stool or flatus.
- 4 - those incontinent to formed stool (complete incontinence).[26]
The fecal incontinence severity index is based on four types of leakage (gas, mucus, liquid stool, solid stool) and five frequencies (once to three times per month, once per week, twice per week, once per day, twice or more per day). Other severity scales include: AMS, Pescatori, Williams score, Kirwan, Miller score, Saint Mark's score and the Vaizey scale.[2]
Differential diagnosis
FI may present with signs similar to rectal discharge (e.g. fistulae, proctitis or rectal prolapse), pseudoincontinence, encopresis (with no organic cause) and irritable bowel syndrome.[2]
Management
| Stool consistency | Cause | First line | Second line |
|---|---|---|---|
| Diarrhea | Inflammatory | Anti-inflammatory drugs | Constipating drugs |
| Pseudodiarrhea | Encopresis | Laxatives | Lavage |
| Solid | Pelvic floor | Biofeedback | Sacral nerve stimulation |
| Sphincter intact | Sacral nerve stimulation | Lavage | |
| Sphincter rupture | Anal repair | Sacral nerve stimulation/Neosphincter | |
| Anal atresia | Lavage | Neosphincter | |
| Rectal prolapse | Rectopexy | Perineal resection | |
| Soiling | Keyhole defect | Lavage | PTQ implant |
FI is generally treatable with conservative management, surgery or both.[2] The success of treatment depends upon the exact causes and how easily these are corrected.[4] Treatment choice depends on the cause and severity of disease, and the motivation and general health of the person effected. Commonly, conservative measures are used together, and if appropriate surgery carried out. Treatments may be attempted until symptoms are satisfactorily controlled. A treatment algorithm based upon the cause has been proposed, including conservative, non-operative and surgical measures (neosphincter refers to either dynamic graciloplasty or artificial bowel sphincter, lavage refers to retrograde rectal irrigation).[2] Conservative measures include dietary modification, drug treatment, retrograde anal irrigation, biofeedback retraining anal sphincter exercises. Incontinence products refer to devices such as anal plugs and perineal pads and garments such as diapers/nappies. Perineal pads are efficient and acceptable for only minor incontinence.[2] If all other measures are ineffective removing the entire colon may be an option.
Diet
Dietary modification may be important for successful management.[3] Both diarrhea and constipation can contribute to different cases, so dietary advice must be tailored to address the underlying cause or it may be ineffective or counter productive. In persons with disease aggravated by diarrhea or those with rectal loading by soft stools, the following suggestions may be beneficial: increase dietary fiber; reduce wholegrain cereals/bread; reduce fruit and vegetables which contain natural laxative compounds (rhubarb, figs, prunes/plums); limit beans, pulses, cabbage and sprouts; reduce spices (especially chilli); reduce artificial sweeteners (e.g. sugar free chewing gum); reduce alcohol (especially stout, beer and ale); reduce lactose if there is some degree of lactase deficiency; and reduce caffeine. Caffeine lowers the resting tone of the anal canal and also causes diarrhea. Excessive doses of vitamin C, magnesium, phosphorus and/or calcium supplements may increase FI. Reducing olestra fat substitute, which can cause diarrhea, may also help.[27]
Medication
Pharmacological management may include anti-diarrheal/constipating agents and laxatives/stool bulking agents Stopping or substituting any previous medication that causes diarrhea may be helpful in some (see table). There is not good evidence for the use of any medications however.[28]
In people who have undergone gallbladder removal, the bile acid sequestrant cholestyramine may help minor degrees of FI.[29] Bulking agents also absorb water, so may be helpful for those with diarrhea. A common side effect is bloating and flatulence. Topical agents to treat and prevent dermatitis may also be used, such as topical antifungals when there is evidence of perianal candidiasis or occasionally mild topical anti-inflammatory medication. Prevention of secondary lesions is carried out by perineal cleansing, moisturization, and use of a skin protectant.[30]
Other measures
Evacuation aids (suppositories or enemas) e.g. glycerine or bisacodyl suppositories may be prescribed. People may have poor resting tone of the anal canal, and consequently may not be able to retain an enema, in which case transanal irrigation (retrograde anal irrigation) may be a better option, as this equipment utilizes an inflatable catheter to prevent loss of the irrigation tip and to provide a water tight seal during irrigation. A volume of lukewarm water is gently pumped into the colon via the anus. People can be taught how to perform this treatment in their own homes, but it does require special equipment. If the irrigation is efficient, stool will not reach the rectum again for up to 48 hours.[31] By regularly emptying the bowel using transanal irrigation, controlled bowel function is often re-established to a high degree in patients with bowel incontinence and/or constipation. This enables control over the time and place of evacuation and development of a consistent bowel routine.[31] However, persistent leaking of residual irrigation fluid during the day may occur and make this option unhelpful, particularly in persons with obstructed defecation syndrome who may have incomplete evacuation of any rectal contents. Consequently, the best time to carry out the irrigation is typically in the evening, allowing any residual liquid to be passed the next morning before leaving the home. Complications such as electrolyte imbalance and perforation are rare. The effect of transanal irrigation varies considerably. Some individuals experience complete control of incontinence, and other report little or no benefit.[31] It has been suggested that if appropriate, people be offered home retrograde anal irrigation.[4]
Biofeedback (the use equipment to record or amplify and then feed back activities of the body) is a commonly used and researched treatment, but the benefits are uncertain.[32] Biofeedback therapy varies in the way it is delivered, but it is unknown if one type has benefits over another.[32]
The role of pelvic floor exercises and anal sphincter exercises in FI is poorly determined. While there may be some benefit they appear less useful than implanted sacral nerve stimulators. These exercises aim to increase the strength of the pelvic floor muscles (mainly levator ani). The anal sphincters are not technically part of the pelvic floor muscle group, but the EAS is a voluntary, striated muscle which therefore can be strengthened in a similar manner. It has not been established whether pelvic floor exercises can be distinguished from anal sphincter exercises in practice by the people doing them. This kind of exercise is more commonly used to treat urinary incontinence, for which there is a sound evidence base for effectiveness. More rarely are they used in FI. The effect of anal sphincter exercises are variously stated as an increase in the strength, speed or endurance of voluntary contraction (EAS).[32]
Electrical stimulation can also be applied to the anal sphincters and pelvic floor muscles, inducing muscle contraction without traditional exercises (similar to transcutaneous electrical nerve stimulation, TENS). The evidence supporting its use is limited, and any benefit is tentative.[33] In light of the above, intra-anal electrical stimulation (using an anal probe as electrode) appears to be more efficacious than intra-vaginal (using a vaginal probe as electrode).[33] Rarely, skin reactions may occur where the electrodes are placed, but these issues typically resolve when the stimulation is stopped. Surgically implanted sacral nerve stimulation may be more effective than exercises, and electrical stimulation and biofeedback may be more effective than exercises or electrical stimulation by themselves.[32] TENS is also sometimes used to treat FI by transcutaneous tibial nerve stimulation.[34]
In a minority of people, anal plugs may be useful for either standalone therapy or in concert with other treatments.[35] Anal plugs (sometimes termed tampons) aim to block involuntary loss of fecal material, and they vary in design and composition.[4] Polyurethane plugs were reported to perform better than those made of polyvinyl-alcohol.[35] Plugs are less likely to help those with frequent bowel movements,[2] and many find them difficult to tolerate.[35]
In women, a device that functions as an inflatable balloon in the vagina, has been approved for use in the United States.[36]
Surgery
Surgery may be carried out if conservative measures alone are not sufficient to control incontinence. There are many surgical options, and their relative effectiveness is debated due to a lack of good quality evidence. The optimal treatment regime may be a both surgical and non-surgical treatments.[37] The surgical options can be considered in four categories: restoration and improvement of residual sphincter function (sphincteroplasty, sacral nerve stimulation, tibial nerve stimulation, correction of anorectal deformity), replacement / imitation of the sphincter or its function (anal encirclement, SECCA procedure, non-dynamic graciloplasty, perianal injectable bulking agents), dynamic sphincter replacement (artificial bowel sphincter, dynamic graciloplasty), antegrade continence enema (Malone procedure), and finally fecal diversion (e.g. colostomy).[1] A surgical treatment algorithm has been proposed. Isolated sphincter defects (IAS/EAS) may be initially treated with sphincteroplasty and if this fails, the person can be assessed for sacral nerve stimulation. Functional deficits of the EAS and/or IAS (i.e. where there is no structural defect, or only limited EAS structural defect, or with neurogenic incontinence) may be assessed for sacral nerve stimulation. If this fails, neosphincter with either dynamic graciloplasty or artificial anal sphincter may be indicated. Substantial muscular and/or neural defects may be treated with neosphincter initially.[6]
Epidemiology
FI is thought to be very common,[1] but much under-reported due to embarrassment. One study reported a prevalence of 2.2% in the general population.[2] It affects people of all ages, but is more common in older adults (but it should not be considered a normal part of aging).[38] Females are more likely to develop it than males (63% of those with FI over 30 may be female).[1] In 2014, the National Center for Health Statistics reported that one out of every six seniors in the U.S. who lived in their own home or apartment had FI. Men and women were equally affected.[39] 45–50% of people with FI have severe physical and/or mental disabilities.[1]
Risk factors include age, female gender, urinary incontinence, history of vaginal delivery (non-Caesarean section childbirth), obesity,[22] prior anorectal surgery, poor general health and physical limitations. Combined urinary and fecal incontinence is sometimes termed double incontinence, and it is more likely to be present in those with urinary incontinence.[40]
Traditionally, FI was thought to be an insignificant complication of surgery, but it is now known that a variety of different procedures are associated with this possible complication, and sometimes at high levels. Examples are midline internal sphincterotomy (8% risk), lateral internal sphincterotomy, fistulectomy, fistulotomy (18-52%), hemorrhoidectomy (33%), ileo-anal reservoir reconstruction, Lower anterior resection, total abdominal colectomy, ureterosigmoidostomy,[22] and anal dilation (Lord's procedure, 0-50%).[41] Some authors consider obstetric trauma to be the most common cause.[42]
History
While the first mention of urinary incontinence occurs in 1500 BC in the Ebers Papyrus, the first mention of FI in a medical context is unknown.[43] For many centuries, colonic irrigation was the only treatment available. Stoma creation was described in 1776, FI associated with rectal prolapse in 1873 and anterior sphincter repair in 1875. During the mid 20th Century, several operations were developed for instances where the sphincters were intact but weakened.[44] Muscle transpositions using the gluteus maximus or the gracilis were devised, but did not become used widely until later. End-to-end sphincteroplasty is shown to have a high failure rate in 1940. In 1971 Parks and McPartlin first describe an overlapping sphincteroplasty procedure. Biofeedback is first introduced in 1974.[45] In 1975, Parks describes post anal repair, a technique to reinforce the pelvic floor and EAS to treat idiopathic cases. Endoanal ultrasound is invented in 1991, which starts to demonstrate the high number of occult sphincter tears following vaginal deliveries. In 1994, the use of an endoanal coil during pelvic MRI shows greater detail of the anal canal than previously. During the last 20 years, dynamic graciliplasty, sacral nerve stimulation, injectable perianal bulking agents and radiofrequency ablation have been devised, mainly due to the relatively poor success rates and high morbidity associated with the earlier procedures.[44]
Society and culture
Persons with this symptom are frequently ridiculed and ostracized in public. It has been described as one of the most psychologically and socially debilitating conditions in an otherwise healthy individual. In older people, it is one of the most common reasons for admission into a care home. Persons who develop FI earlier in life are less likely to marry and obtain employment. Often, people will go to great lengths to keep their condition secret. It has been termed "the silent affliction" since many do not discuss the problem with their close family, employers or clinicians. They may be subject to gossip, hostility, and other forms of social exclusion.[46][47][48] The economic cost has not received much attention. In the Netherlands, outpatients were reported to have total costs of €2169 annually, and over half of this was productivity loss in work. In the USA, the average lifetime cost (treatment and follow-up) was $17,166 per person in 1996. The average hospital charges for sphincteroplasty was $8555 per procedure. Overall, in the USA, the total charges associated with surgery increased from $34 million in 1998 to $57.5 million in 2003. Sacral nerve stimulation, dynamic graciloplasty and colostomy were all shown to be cost effective.[49]
Japan
Among the most offensive insults in Japan (on the same level as fuck in English) relate to incontinence, such as kusotare/kusottare and shikkotare which mean shit hanger/leaker/oozer and piss leaker/oozer respectively.[50]
Research
Engineered anal sphincters grown from stem cells have been successfully implanted in mice. New blood vessels developed and the tissue displayed normal contraction and relaxation. In the future, these methods may become part of the management of FI, replacing the need for high morbidity implanted devices such as the artificial bowel sphincter.[51]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 Kaiser, Andreas M. "ASCRS core subjects: fecal incontinence". ASCRS. Retrieved 29 October 2012.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 Bruce G. Wolff et al., eds. (2007). The ASCRS textbook of colon and rectal surgery. New York: Springer. pp. 653–664. ISBN 0-387-24846-3.
- 1 2 3 4 5 6 7 8 9 10 Tadataka Yamada, David H. Alpers, et al., eds. (2009). Textbook of gastroenterology (5th ed.). Chichester, West Sussex: Blackwell Pub. pp. 1717–1744. ISBN 978-1-4051-6911-0.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 (UK), National Collaborating Centre for Acute Care (2007). Faecal incontinence the management of faecal incontinence in adults. London: National Collaborating Centre for Acute Care (UK). ISBN 0-9549760-4-5.
- ↑ Paul Abrams et al., eds. (2009). "Pathophysiology of Urinary Incontinence, Faecal Incontinence and Pelvic Organ Prolapse". Incontinence : 4th International Consultation on Incontinence, Paris, July 5-8, 2008 (4th ed.). [Paris]: Health Publications. p. 255. ISBN 0-9546956-8-2.
- 1 2 3 Wexner, edited by Andrew P. Zbar, Steven D. (2010). Coloproctology. New York: Springer. pp. 109–119. ISBN 978-1-84882-755-4.
- ↑ Nusrat, S; Gulick, E; Levinthal, D; Bielefeldt, K (2012). "Anorectal dysfunction in multiple sclerosis: a systematic review.". ISRN neurology. 2012: 376023. PMC 3414061
 . PMID 22900202. doi:10.5402/2012/376023.
. PMID 22900202. doi:10.5402/2012/376023. - ↑ Rodrigues, ML; Motta, ME (Jan–Feb 2012). "Mechanisms and factors associated with gastrointestinal symptoms in patients with diabetes mellitus.". Jornal de pediatria. 88 (1): 17–24. PMID 22344626. doi:10.2223/jped.2153.
- ↑ Hoffmann BA, Timmcke AE, Gathright JB, Hicks TC, Opelka FG, Beck DE (July 1995). "Fecal seepage and soiling: a problem of rectal sensation.". Diseases of the colon and rectum. 38 (7): 746–8. PMID 7607037. doi:10.1007/bf02048034.
- ↑ Burgell, Rebecca E; Scott, S Mark (October 2012). "Rectal Hyposensitivity". Journal of Neurogastroenterology and Motility. 18 (4): 373–84. PMC 3479250
 . PMID 23105997. doi:10.5056/jnm.2012.18.4.373.
. PMID 23105997. doi:10.5056/jnm.2012.18.4.373. - ↑ Rao, SS (January 2004). "Pathophysiology of adult fecal incontinence.". Gastroenterology. 126 (1 Suppl 1): S14–22. PMID 14978634. doi:10.1053/j.gastro.2003.10.013.
- ↑ Rao, SS; Ozturk, R; Stessman, M (November 2004). "Investigation of the pathophysiology of fecal seepage.". The American journal of gastroenterology. 99 (11): 2204–9. PMID 15555003. doi:10.1111/j.1572-0241.2004.40387.x.
- ↑ (UK), National Collaborating Centre for Acute Care (2007). "Appendix J". Faecal incontinence the management of faecal incontinence in adults. London: National Collaborating Centre for Acute Care (UK). ISBN 0-9549760-4-5.
- ↑ Salat-Foix, D; Suchowersky, O (February 2012). "The management of gastrointestinal symptoms in Parkinson's disease.". Expert Review of Neurotherapeutics. 12 (2): 239–48. PMID 22288679. doi:10.1586/ern.11.192.
- ↑ Bromfield, EB; Cavazos, JE; Sirven, JI (2006). "An Introduction to Epilepsy". PMID 20821849.
- ↑ Nallamshetty, L; Ahn, NU; Ahn, UM; Nallamshetty, HS; Rose, PS; Buchowski, JM; Sponseller, PD (August 2002). "Dural ectasia and back pain: review of the literature and case report.". Journal of spinal disorders & techniques. 15 (4): 326–9. PMID 12177551. doi:10.1097/00024720-200208000-00012.
- ↑ Kang, Jun Goo; Park, Cheol-Young (1 January 2012). "Anti-Obesity Drugs: A Review about Their Effects and Safety". Diabetes & Metabolism Journal. 36 (1): 13–25. PMC 3283822
 . PMID 22363917. doi:10.4093/dmj.2012.36.1.13.
. PMID 22363917. doi:10.4093/dmj.2012.36.1.13. - ↑ Reginelli A, Di Grezia G, Gatta G, Iacobellis F, Rossi C, Giganti M, Coppolino F, Brunese L (2013). "Role of conventional radiology and MRi defecography of pelvic floor hernias.". BMC Surgery. 13 Suppl 2: S53. PMC 3851064
 . PMID 24267789. doi:10.1186/1471-2482-13-S2-S53.
. PMID 24267789. doi:10.1186/1471-2482-13-S2-S53. - ↑ Abdool, Z; Sultan, AH; Thakar, R (July 2012). "Ultrasound imaging of the anal sphincter complex: a review.". The British journal of radiology. 85 (1015): 865–75. PMID 22374273. doi:10.1259/bjr/27314678.
- ↑ Bharucha, AE; Wald, A; Enck, P; Rao, S (April 2006). "Functional anorectal disorders.". Gastroenterology. 130 (5): 1510–8. PMID 16678564. doi:10.1053/j.gastro.2005.11.064.
- ↑ "Rome III Diagnostic Criteria for Functional Gastrointestinal Disorders". Rome Foundation. Retrieved 3 November 2012.
- 1 2 3 Paul Abrams et al., eds. (2009). "Epidemiology of Urinary (UI) and Faecal (FI) Incontinence and Pelvic Organ Prolapse (POP)". Incontinence : 4th International Consultation on Incontinence, Paris, July 5-8, 2008 (PDF) (4th ed.). [Paris]: Health Publications. p. 35. ISBN 0-9546956-8-2. PMID 20025020.
- ↑ Kaneshiro, Neil. "Encopresis". Medline Plus. Retrieved 2 July 2012.
- ↑ "ICD-10 Classification of "Nonorganic encopresis"". World Health Organization. Retrieved 4 February 2013.
- ↑ Shamliyan, TA; Bliss, DZ; Du, J; Ping, R; Wilt, TJ; Kane, RL (Fall 2009). "Prevalence and risk factors of fecal incontinence in community-dwelling men.". Reviews in gastroenterological disorders. 9 (4): E97–110. PMID 20065920.
- ↑ Fecal Incontinence: Diagnosis and Treatment, p. 91, at Google Books
- ↑ Food/drink which may Exacerbate Faecal Incontinence in Patients who Present with Loose Stools or Rectal Loading of Soft Stool 2007. National Collaborating Centre for Acute Care.
- ↑ Omar, MI; Alexander, CE (11 June 2013). "Drug treatment for faecal incontinence in adults.". The Cochrane database of systematic reviews. 6: CD002116. PMID 23757096. doi:10.1002/14651858.CD002116.pub2.
- ↑ Romano, [edited by] Carlo Ratto, Giovanni B. Doglietto ; forewords by A.C Lowry, L. Paahlman, G. (2007). Fecal incontinence : diagnosis and treatment (1. ed.). Milan: Springer. p. 313. ISBN 88-470-0637-6.
- ↑ Gray, M; Beeckman, D; Bliss, DZ; Fader, M; Logan, S; Junkin, J; Selekof, J; Doughty, D; Kurz, P (Jan–Feb 2012). "Incontinence-associated dermatitis: a comprehensive review and update.". Journal of wound, ostomy, and continence nursing : official publication of The Wound, Ostomy and Continence Nurses Society / WOCN. 39 (1): 61–74. PMID 22193141. doi:10.1097/WON.0b013e31823fe246.
- 1 2 3 Emmanuel AV, Krogh K, Bazzocchi G, Leroi AM, Bremers A, Leder D, van Kuppevelt D, Mosiello G, Vogel M, Perrouin-Verbe B, Coggrave M, Christensen P (Oct 2013). "Consensus review of best practice of transanal irrigation in adults." (PDF). Spinal Cord. 51 (10): 732–8. PMID 23958927. doi:10.1038/sc.2013.86.
- 1 2 3 4 Norton, C; Cody, JD (Jul 11, 2012). "Biofeedback and/or sphincter exercises for the treatment of faecal incontinence in adults.". Cochrane database of systematic reviews (Online). 7: CD002111. PMID 22786479. doi:10.1002/14651858.CD002111.pub3.
- 1 2 Hosker, G; Cody, JD; Norton, CC (Jul 18, 2007). "Electrical stimulation for faecal incontinence in adults.". Cochrane database of systematic reviews (Online) (3): CD001310. PMID 17636665. doi:10.1002/14651858.CD001310.pub2.
- ↑ Interventional procedure guidance 395: Percutaneous tibial nerve stimulation for faecal incontinence (PDF). National Institute for Health and Clinical Excellence. May 2011. ISBN 9781849365918.
- 1 2 3 Deutekom, Marije; Dobben, Annette C. (2015-07-20). "Plugs for containing faecal incontinence". The Cochrane Database of Systematic Reviews (7): CD005086. ISSN 1469-493X. PMID 26193665. doi:10.1002/14651858.CD005086.pub4.
- ↑ "FDA permits marketing of fecal incontinence device for women". fda.gov. February 12, 2015. Retrieved 17 February 2015.
- ↑ Brown, SR; Wadhawan, H; Nelson, RL (2 July 2013). "Surgery for faecal incontinence in adults.". The Cochrane database of systematic reviews. 7: CD001757. PMID 23821339. doi:10.1002/14651858.CD001757.pub4.
- ↑ Shah, BJ; Chokhavatia, S; Rose, S (November 2012). "Fecal Incontinence in the Elderly: FAQ.". The American journal of gastroenterology. 107 (11): 1635–46. PMID 22964553. doi:10.1038/ajg.2012.284.
- ↑ Judith Graham (July 29, 2014). "An 'Emotional Burden' Rarely Discussed". New York Times. Retrieved August 23, 2014.
- ↑ Lacima, G; Pera, M (October 2003). "Combined fecal and urinary incontinence: an update.". Current Opinion in Obstetrics and Gynecology. 15 (5): 405–10. PMID 14501244. doi:10.1097/01.gco.0000094702.87578.3b.
- ↑ Ommer, A; Wenger, FA; Rolfs, T; Walz, MK (November 2008). "Continence disorders after anal surgery--a relevant problem?". International journal of colorectal disease. 23 (11): 1023–31. PMID 18629515. doi:10.1007/s00384-008-0524-y.
- ↑ Rieger, N; Wattchow, D (March 1999). "The effect of vaginal delivery on anal function.". The Australian and New Zealand journal of surgery. 69 (3): 172–7. PMID 10075354. doi:10.1046/j.1440-1622.1999.01517.x.
- ↑ Briel, Johan Willem (2000). "1". Treatment of faecal incontinence. [S.l.]: [The Author]. pp. 10–12. ISBN 90-90-13967-2.
- 1 2 Paul Abrams et al., eds. (2009). "Surgery for fecal incontinence". Incontinence : 4th International Consultation on Incontinence, Paris, July 5-8, 2008 (PDF) (4th ed.). [Paris]: Health Publications. pp. 1387, 1567. ISBN 0-9546956-8-2.
- ↑ Engel, BT; Nikoomanesh, P; Schuster, MM (Mar 21, 1974). "Operant conditioning of rectosphincteric responses in the treatment of fecal incontinence.". The New England Journal of Medicine. 290 (12): 646–9. PMID 4813725. doi:10.1056/NEJM197403212901202.
- ↑ Norton, Nancy J. "Barriers on Diagnosis and Treatment; Impact of Fecal and Urinary Incontinence on Health Consumers – Barriers on Diagnosis and Treatment – A Patient Perspective". International Foundation for Functional Gastrointestinal Disorders (IFFGD). Retrieved 1 January 2013.
- ↑ Ranganath, Sonia; Tanaz R Ferzandi. "Fecal Incontinence". WebMD LLC. Retrieved 1 January 2013.
- ↑ Bliss, DZ; Norton, C (September 2010). "Conservative management of fecal incontinence.". The American journal of nursing. 110 (9): 30–8; quiz 39–40. PMID 20736708. doi:10.1097/01.NAJ.0000388262.72298.f5.
- ↑ Paul Abrams et al., eds. (2009). "Economics of urinary and faecal incontinence, and prolapse". Incontinence : 4th International Consultation on Incontinence, Paris, July 5-8, 2008 (4th ed.). [Paris]: Health Publications. p. 1685. ISBN 0-9546956-8-2.
- ↑ Wilson, Scott. "W.T.F. Japan: Top 5 most offensive Japanese swear words 【Weird Top Five】". SoraNews24. Retrieved 12 May 2017.
- ↑ Koch, Kenneth L (1 January 2012). "Tissue engineering for neuromuscular disorders of the gastrointestinal tract". World Journal of Gastroenterology. 18 (47): 6918–25. PMC 3531675
 . PMID 23322989. doi:10.3748/wjg.v18.i47.6918.
. PMID 23322989. doi:10.3748/wjg.v18.i47.6918.