Borenium ion
A borenium ion in chemistry is a trivalent boron cation of the type R1R2BL where R is a substituent and L a ligand. It is one of several boron cations: borinium cations are divalent boron cations with the general structure R1R2B and boronium cations are tetravalent (R1R2BL1L2) with two ligands. The positive charge can reside on the ligand itself or with a ligand to boron dative bond on the boron atom. Borenium ions are of some interest to academic research.[1][2][3]
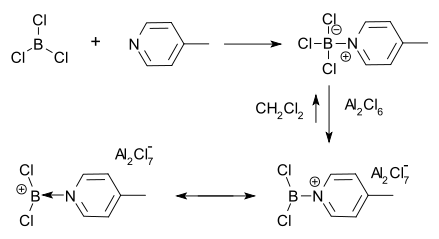
The first borenium ion was observed in 1970 by Ryschkewitsch & Wiggins.[4] They found that aluminum chloride could only dissolve in methylene chloride in the presence of the adduct of 4-picoline and boron trichloride. A positive charge on boron was then inferred from proton NMR.
Related boron cations
Borinium ions have the formula [BX2]+, where X− is usual a bulky amide (R2N−). Such species have linear geometry at boron and are coordinatively unsaturated. Boronium ions have the formula [L2BR2]+ (L = Lewis base). A well known example is (H3N)2BH2]+. Together with ammonia borane, this ion is produced by treating borane with ammonia. Boronium ions are tetrahedral and coordinatively saturated.[3][2]
References
- ↑ The chemistry of borinium and borenium ions P. Koelle and H. Noeth Chemical Reviews 1985 85 (5), 399-418 doi:10.1021/cr00069a004
- 1 2 Piers, W. E., Bourke, S. C. and Conroy, K. D. (2005), Borinium, Borenium, and Boronium Ions: Synthesis, Reactivity, and Applications. Angew. Chem. Int. Ed., 44: 5016–5036. doi:10.1002/anie.200500402
- 1 2 Timothy S. De Vries, Aleksandrs Prokofjevs, Edwin Vedejs Cationic Tricoordinate Boron Intermediates: Borenium Chemistry from the Organic Perspective Chemical Reviews 2012, volume 112, 4246-4282 doi:10.1021/cr200133c
- ↑ Trigonal boron cation George E. Ryschkewitsch and J. W. Wiggins Journal of the American Chemical Society 1970 92 (6), 1790-1791 doi:10.1021/ja00709a079