Borinic acid
| Names | |
|---|---|
| Other names
Boranol, Hydroxyborane, Dihydridohydroxidoboron | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChEBI | |
| 141192 | |
| PubChem CID |
|
| |
| |
| Properties | |
| BH3O | |
| Molar mass | 29.83 g·mol−1 |
| reaction in water | |
| Structure | |
| distorted trigonal bipyramid | |
| Related compounds | |
| Related compounds |
Boronic acid Boric acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |

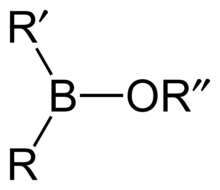
Borinic acid H
2BOH, also known as boronous acid, is an oxyacid of boron with formula H
2BOH. Borinate is the associated anion of boron with formula H
2BO−
, however being a lewis acid the form in basic solution is H
2B(OH)−
2.
Borinic acid can be formed as the first step in the hydrolysis of diborane.[1] BH3+H2O → H2BOH + H2 Borinic acid itself is unstable and only lasts for a few seconds during the hydrolysis reaction. However, by using microwave spectroscopy various properties can be determined. The B-O distance is 1.352 Å, O-H distance 0.96 Å, B-H length is probably 1.2 Å. The angle between bonds at the oxygen atom BOH = 112° and the angles at boron are cis-HBO 121°, and trans-HBO = 117°. The dipole moment is 1.506 Debye.[2]
Borinic acid can form esters such as methoxyborane. This too is unstable only lasting about ten seconds. It can be formed by heating diborane and methanol gas together.[3]
By substituting organic components instead of hydrogen, more generic borinic acids (containing RR'BOH) or borinic esters (RR'BOR") can be formed. Esters will tend to be stable in acidic conditions, but in alkaline conditions the boron atom can gain a negative charge and attach two hydroxyl groups, or two ester bonds. RR'B−(OH)2 or RR'B−(OR")2. The anionic borinate ion can very easily form esters with diols such as ethylene glycol or sugars.[4]
Naming
IUPAC naming for Borinic acid is a unique name for acid.[5] The anhydrides are named diboroxanes, H2BOBH2, as the base compound and H being able to be substituted, e.g. tetraethyldiboroxane, as the anhydride for diethylborinic acid. Organic naming standard in the bluebook allows skeletal replacement naming where the name is shorter, 3-borapentan-3-ol versus diethyl borinic acid. The grouping -BH-O-BH2 is called diboroxanyl. Substituting sulfur for oxygen gives borinothioic acid (H2BSH). (dimethylboranyl)oxy is used for the group (CH3)2B-O− and methyl(hydroxy)boranyl for the grouping CH3B(OH)-.
Formation
There are several ways to produce substituted borinic acids.[6]
Firstly borinic acids can be made from oxidising trialkyl borane starting materials [R3B] with exposure to moist air, or treatment with iodine, which makes a dialkyliodoborane [R2BI]. Hydrolysis then results in the boronic acid (R2BOH).[6] Trialkylborates [(RO)3B] or trialkoxyboroxine [(ROBO)3] can be reduced to borinic acid by us of a Grignard reagent. Grignard reagents can also reduce a boronic ester [RB(OR')2] to a borinic ester.[6]
Bu3B + N2CHCOR → BuCH=C(R)OBBu2
Bu3B + CH2=CHCOCH3 → BuCH2CH=C(CH3)OBBu2
RCOC2H5 + R2BOTf → RC(OBR2)=CHCH3
(Tf = Trifluoromethanesulfonate)
[Z] enolate gives syn aldol when reacted with aldehyde, where as [E] enolate gives and anti aldol
Dialkyl boron chloride (R2BCL) with tertiary amine react with ketones to form an enol borinate.[7]
A trialkoxyborane can react with lithium containing organic molecules to eliminate lithium and one or two alkoxy groups to make boronic and borinic esters.[8]
Purification of the mixtures that result from the reactions is required, as often boronic esters will also be produced and mixed in with the borinic esters.[6] The method of Letsinger is dissolve the mixture in ether and precipitate the borinic ester by forming a complex with ammonia. Treatment with ethanolamine ends up making an aminoetylborinate.[6]
Compounds
| R2BOR' | borinic acid R'=H | anhydride | esters R' | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | hydrogen | -O- | aminoethyl | ethyl | n-propyl | n-butyl | 3-methylpropyl | 1-methylpropyl | phenyl | ethylene glycol | methyl | 8-quinolinyl |
| phenyl | [9] | SID 535455 | ||||||||||
| o-tolyl | ||||||||||||
| m-tolyl | ||||||||||||
| p-tolyl | ||||||||||||
| p-anisyl | ||||||||||||
| p-biphenyl | ||||||||||||
| p-chlorophenyl | ||||||||||||
| 3-chlorophenyl | ||||||||||||
| α-naphthyl | ||||||||||||
| β-naphthyl | ||||||||||||
| p-bromophenyl | ||||||||||||
| 2-methyl-5-chlorophenyl | ||||||||||||
| 2-thienyl | [17] SID 3881207 | SID 8142470 | ||||||||||
| mesityl | sid 4278417 CAS 20631-84-9[18][19][20] | |||||||||||
| methyl | ||||||||||||
| ethyl | ||||||||||||
| allyl | ||||||||||||
| n-butyl | cas 19324-14-2 | |||||||||||
| 4-methylbutyl | ||||||||||||
| 2-chlorovinyl | ||||||||||||
| 3,5-dimethylphenyl | ||||||||||||
| propyl | ||||||||||||
| 1-methylpropyl | cas 4026-69-1 | |||||||||||
| 2-methylpropyl | cas 4026-82-8 | |||||||||||
2-APB

2-aminoethyl-diphenylborinate also known as 2-APB, inhibits transient receptor potential channels.[25] This kind of inhibition is being researched to find treatments for prostate cancer. In particular TRPM7. 2-APB can work as a catalyst to add an alkyl group from an alkyl halide to a polyol or carbohydrate that contains a cis-vicinal diol to a precise position. It does this by first combining with the two hydroxy groups to make a ring containing OCCOB−.[26] It can also calatlyse acid chloride or chloroformate reaction a specific region of the diol.[27]
Diphenylborinic acid
Diphenylborinic acid was discovered in 1894 by Michaelis who produced it by hydrolysing the chloride. Letsinger determined its properties in 1955.[6]
Diphenylborinic acid has an extra high affinity for catechols compared with carbohydrates[28]
Diphenylborinic acid can catalyse the condensation of pyruvic acids with aldehydes to yield substituted isotetronic acid.[29]
Diphenylborinic acid is an inhibitor of several enzymes such as α-chymotrypsin, subtilisin BPN' and trypsin.[30]
Borinate radical
Borinate radicals (RR'BO·) can be formed from peroxyborinate decomposition.[31]
Other compounds
Other compounds include methoxy(dimethyl)borane, methoxy(methyl)boron, methoxy(methylidene)borane (with a C=B double bond).[32]
[C5H5BR]− uses a B− to be equivalent to carbon in an aromatic benzene like ring. This too is called borinate. The 1-methyl and 1-phenyl borinates can form some of the few organo-thallium(I) compounds.[33]
HB(C6F5)2 + phosphino alcohol → tBu2P+HCH2C(CH3)2OB−H(C6F5)2 → H2 + tBu2PCH2C(CH3)2OB(C6F5)2 and same for tBu2PCH2C(CF3)2OB(C6F5)2[34]
di-Tris(tert-butoxy)siloxy borinic acid HOB[OSi(O(t)Bu)3]2 can be made from tributoxyborate and tributoxysiloxane. It can form a very complex crystal with Cp2Zr(Me)[OB[OSi(O(t)Bu)3]2]2.[35]
Diborinic acids have two RBOH groups linked together by an organic connection such as diphenyl or phenyl.[36]
Applications
1,1,1,3,3,3-Hexafluoroisopropylbis(pentafluorophenyl)borinate can greatly increase solubility of LiF by complexing the F− anion.[37] This has potential to improve lithium batteries.
Borinic esters are being researched as bacterial growth inhibitors[38] due to their ability to disable some bacterial enzymes such as menaquinone methyltransferase and CcrM.[39] This may result in development of treatments for topical application on skin.[40]
References
- ↑ Weiss, H. G.; Shapiro, I. (5 March 1953). "Mechanism of the Hydrolysis of Diborane in the Vapor Phase1". Journal of the American Chemical Society. 75 (5): 1221–1224. doi:10.1021/ja01101a061.
- ↑ Kawashima, Yoshiyuki; Takeo, Harutoshi; Matsumura, Chi (1 January 1981). "Microwave spectrum of borinic acid BH2OH". The Journal of Chemical Physics. 74 (10): 5430. Bibcode:1981JChPh..74.5430K. doi:10.1063/1.440947.
- ↑ Kawashima, Y.; Takeo, H.; Matsumura, C. "Microwave Spectrum of Methoxyborane, CH3OBH2". Retrieved 14 October 2013.
- ↑ Pappin, Brighid; Milton J. Kiefel; Todd A. Houston (2012). "Boron Carbohydrate Interactions". Carbohydrates - Comprehensive Studies on Glycobiology and Glycotchnology. ISBN 978-953-51-0864-1. doi:10.5772/50630.
- ↑ Connelly, Neil G.; Damhus, Ture; Hartshorn, Richard M.; Alan T. Hutton (2005). "Table IR-8.1 Acceptable common names". Nomenclature of Inorganic Chemistry. International Union of Pure and Applied Chemistry. p. 127. ISBN 0-85404-438-8.
- 1 2 3 4 5 6 Povlock, Thomas Paul (August 1960). "Boroxines: Their Reactions and Ring Stability" (PDF). Retrieved 5 October 2013.
- ↑ Brown, Herbert C; Rhaj K. Dhar (March 16, 1989). "Major Effect of the Leaving Group in Dialkyl Boron Chlorides and Triflates in COntrolling the Stereoscopic COnversion of Ketones into Either [E] or [Z]- Enol Borinates" (PDF). Retrieved 25 September 2013.
- ↑ Delbrayelle, Dominique (15 June 2010). "Boronic Acids Manufacture at Industrial Scale" (PDF). Retrieved 5 October 2013.
- ↑ "Crystal structure of tetraphenyldiboroxane a monomer diboroxane". Inorganic Chemistry Communications. 5: 377–379. doi:10.1016/S1387-7003(02)00402-1.
- ↑ Erbes, Michael; Klaus Forstinger; Andreas Meudt (31 Oct 2002). "Process for preparing boronic and borinic acids". US Patent 20020161230. Retrieved 15 October 2013.
- 1 2 3 Povlock, Thomas Paul (August 1960). "Boroxines: Their Reactions and Ring Stability" (PDF). Retrieved 5 October 2013. ref 24
- ↑ Povlock, Thomas Paul (August 1960). "Boroxines: Their Reactions and Ring Stability" (PDF). Retrieved 5 October 2013. ref 25
- 1 2 Povlock, Thomas Paul (August 1960). "Boroxines: Their Reactions and Ring Stability" (PDF). Retrieved 5 October 2013. ref 26
- ↑ Povlock, Thomas Paul (August 1960). "Boroxines: Their Reactions and Ring Stability" (PDF). Retrieved 5 October 2013. ref 5
- ↑ Povlock, Thomas Paul (August 1960). "Boroxines: Their Reactions and Ring Stability" (PDF). Retrieved 5 October 2013. ref 9
- ↑ Povlock, Thomas Paul (August 1960). "Boroxines: Their Reactions and Ring Stability" (PDF). Retrieved 5 October 2013. ref 2
- ↑ Low, John Nicolson; Musgrave, Oliver; Wardell, James (15 February 2000). "(2-Aminoethoxy)bis(2-thienyl)boron". Acta Crystallographica Section C. 56 (2): e63–e63. doi:10.1107/S0108270100000482.
- ↑ Birch, Samuel J.; Boss, Sally R.; Cole, Sarah C.; Coles, Martyn P.; Haigh, Robert; Hitchcock, Peter B.; Wheatley, Andrew E. H. (1 January 2004). "The structural characteristics of organozinc complexes incorporating N,N?-bidentate ligands". Dalton Transactions (21): 3568. doi:10.1039/B410945G.
- ↑ Kuhlmann, Matthias; Baumgartner, Thomas; Parvez, Masood (7 June 2008). "A new polymorph of dimesitylborinic acid". Acta Crystallographica Section E. 64 (7): o1185–o1185. doi:10.1107/S1600536808015638.
- ↑ Weese, Kenneth J.; Bartlett, Ruth A.; Murray, Brendan D.; Olmstead, Marilyn M.; Power, Philip R. (1 July 1987). "Synthesis and spectroscopic and structural characterization of derivatives of the quasi-alkoxide ligand [OBMes2]- (Mes = 2,4,6-Me3C6H2)". Inorganic Chemistry. 26 (15): 2409–2413. doi:10.1021/ic00262a015.
- ↑ Povlock, Thomas Paul (August 1960). "Boroxines: Their Reactions and Ring Stability" (PDF). Retrieved 5 October 2013. ref 28
- ↑ Povlock, Thomas Paul (August 1960). "Boroxines: Their Reactions and Ring Stability" (PDF). Retrieved 5 October 2013. ref 17
- ↑ Povlock, Thomas Paul (August 1960). "Boroxines: Their Reactions and Ring Stability" (PDF). Retrieved 5 October 2013. ref 29
- ↑ Winkle, D. (2001). "boronic acids can be used as viable alternatives to borinic acids in suzuki coupling reactions". Organic Process Research and Deve1opment. 5 (4): 450–451. doi:10.1021/op010207s.
- ↑ Carboxylic Acids—Advances in Research and Application. 2012. p. 93. ISBN 9781464993923.
- ↑ Chan, Lina; Taylor, Mark S. (17 June 2011). "Regioselective Alkylation of Carbohydrate Derivatives Catalyzed by a Diarylborinic Acid Derivative". Organic Letters. 13 (12): 3090–3093. PMID 21591630. doi:10.1021/ol200990e.
- ↑ Lee, Doris; Taylor, Mark S. (23 March 2011). "Borinic Acid-Catalyzed Regioselective Acylation of Carbohydrate Derivatives". Journal of the American Chemical Society. 133 (11): 3724–3727. PMID 21355584. doi:10.1021/ja110332r.
- ↑ Chudzinski, Michael G.; Chi, Yuechuan; Taylor, Mark S. (1 January 2011). "Borinic Acids: A Neglected Class of Organoboron Compounds for Recognition of Diols in Aqueous Solution". Australian Journal of Chemistry. 64 (11): 1466. doi:10.1071/CH11294.
- ↑ Lee, Doris; Newman, Stephen G.; Taylor, Mark S. (3 December 2009). "Boron-Catalyzed Direct Aldol Reactions of Pyruvic Acids". Organic Letters. 11 (23): 5486–5489. PMID 19904926. doi:10.1021/ol902322r.
- ↑ Steiner, Steven J.; Bien, Jeffrey T.; Smith, Bradley D. (1 October 1994). "Diphenylborinic acid is a strong inhibitor of serine proteases". Bioorganic & Medicinal Chemistry Letters. 4 (20): 2417–2420. doi:10.1016/S0960-894X(01)80401-7.
- ↑ Robin Hicks (2 August 2011). Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds. John Wiley and Sons. p. 518. ISBN 9781119956969.
- ↑ "Similar compounds". Retrieved 4 October 2013.
- ↑ Janiac (1997). "(Organo) thallium (I) and (II) chemistry: syntheses, structures, properties and applications of subvalent thallium complexes with alkyl, cyclopentadienyl, arene or hydrotris (pyrazolyl) borate ligands" (PDF). Coordination Chemistry Reviews. 163: 124. doi:10.1016/s0010-8545(97)00011-8.
- ↑ Chapman, Andy M.; Haddow, Mairi F.; Orton, Jonathan P. H.; Wass, Duncan F. (1 January 2010). "Facile dihydrogen release from phosphino-borinate ester Lewis pairs". Dalton Transactions. 39 (27): 6184. doi:10.1039/c0dt00513d.
- ↑ Fujdala, KL; Oliver, A. G.; Hollander, F. J.; Tilley, T. D. (Feb 24, 2003). "Tris(tert-butoxy)siloxy derivatives of boron, including the boronous acid HOB[OSi(O(t)Bu)(3)](2) and the metal (siloxy)boryloxide complex Cp(2)Zr(Me)OB[OSi(O(t)Bu)(3)](2): a remarkable crystal structure with 18 independent molecules in its asymmetric unit". Inorganic Chemistry. 42 (4): 1140–1150. PMID 12588150. doi:10.1021/ic0205482.
- ↑ Suzuki, Akinobu Z.; Shoichiro Ozaki, Jun-Ichi Goto Katsuhiko Mikishiba (15 Feb 2010). "Synthesis of bisboron Compounds and their Strong Inhibitory Activity on Store-operated Calcium Entry". Bioinorganic and Medicinal Chemistry Letters. 20 (4): 1395–1398. doi:10.1016/j.bmcl.2009.12.108.
- ↑ McBreen, J.; H. S. Lee; X. Q. Yang (2003). "Borinates: A New Family of Anion Complexing Agents" (PDF). The Electrochemical Society, Inc. Retrieved 25 September 2013.
- ↑ Bailey, P. J.; G Cousins; G A Snow; A J White (April 1980). "Boron-containing antibacterial agents: effects on growth and morphology of bacteria under various culture conditions" (PDF). Antimicrobial Agents and Chemotherapy. American Society for Microbiology. 17 (4): 549–553. PMC 283830
 . PMID 6994634. doi:10.1128/aac.17.4.549. Retrieved 5 October 2013.
. PMID 6994634. doi:10.1128/aac.17.4.549. Retrieved 5 October 2013. - ↑ Benkovic SJ, Baker SJ, Alley MR, Woo YH, Zhang YK, Akama T, Mao W, Baboval J, Rajagopalan PT, Wall M, Kahng LS, Tavassoli A, Shapiro L (1 November 2005). "Identification of Borinic Esters as Inhibitors of Bacterial Cell Growth and Bacterial Methyltransferases, CcrM and MenH". Journal of Medicinal Chemistry. 48 (23): 7468–7476. PMID 16279806. doi:10.1021/jm050676a.
- ↑ Baker SJ, Akama T, Zhang YK, Sauro V, Pandit C, Singh R, Kully M, Khan J, Plattner JJ, Benkovic SJ, Lee V, Maples KR (2006). "Identification of a novel boron-containing antibacterial agent (AN0128) with anti-inflammatory activity, for the potential treatment of cutaneous diseases". Bioorg. Med. Chem. Lett. 16 (23): 5963–7. PMID 16997550. doi:10.1016/j.bmcl.2006.08.130.
| Wikimedia Commons has media related to Borinate esters. |
Extra reading
- Dimitrijević, Elena; Taylor, Mark S. (3 May 2013). "Organoboron Acids and Their Derivatives as Catalysts for Organic Synthesis". ACS Catalysis. 3 (5): 945–962. doi:10.1021/cs4000848. Review of use borinic acids as a catalyst
- Roth, HJ; Miller, B (September 1964). "Synthesis of Phenyl- and Thienyl-Substituted Boronic and Borinic Acids.". Archiv der Pharmazie. 297: 513–523. PMID 14341914. doi:10.1002/ardp.19642970902.