Biogasoline
Biogasoline is gasoline produced from biomass such as algae. Like traditionally produced gasoline, it contains between 6 (hexane) and 12 (dodecane) carbon atoms per molecule and can be used in internal-combustion engines. Biogasoline is chemically different from biobutanol and bioethanol, as these are alcohols, not hydrocarbons.
Companies such as Diversified Energy Corporation are developing approaches to take triglyceride inputs and through a process of deoxygenation and reforming (cracking, isomerizing, aromatizing, and producing cyclic molecules) producing biogasoline. This biogasoline is intended to match the chemical, kinetic, and combustion characteristics of its petroleum counterpart, but with much higher octane levels. Others are pursuing similar approaches based on hydrotreating. And lastly still others are focused on the use of woody biomass for conversion to biogasoline using enzymatic processes.
Structure and properties
BG100, or 100% biogasoline, can immediately be used as a drop-in substitute for petroleum gasoline in any conventional gasoline engine, and can be distributed in the same fueling infrastructure, as the properties match traditional gasoline from petroleum.[1] Dodecane requires a small percentage of octane booster to match gasoline. Ethanol fuel (E85) requires a special engine and has lower combustion energy and corresponding fuel economy.[2]
But due to biogasoline's chemical similarities it can also be mixed with regular gasoline. You can have higher ratios of biogasoline to gasoline and not have to modify the vehicles engine unlike ethanol.[3]
Comparison to common fuels
| Fuel | Energy Density MJ/L |
Air-fuel ratio |
Specific Energy MJ/kg |
Heat of Vaporization MJ/kg |
RON | MON |
|---|---|---|---|---|---|---|
| Gasoline | 34.6 | 14.6 | 46.9 | 0.36 | 91–99 | 81–89 |
| Butanol fuel | 29.2 | 11.2 | 36.6 | 0.43 | 96 | 78 |
| Ethanol fuel | 24.0 | 9.0 | 30.0 | 0.92 | 129 | 102 |
| Methanol fuel | 19.7 | 6.5 | 15.6 | 1.2 | 136 | 104 |
Production
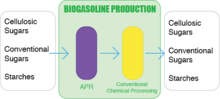
Biogasoline is created by turning sugar directly into gasoline. In late March 2010, the world’s first biogasoline demonstration plant was started in Madison, WI by Virent Energy Systems, Inc.[4] Virent discovered and developed a technique called Aqueous Phase Reforming (APR) in 2001. APR includes many processes including reforming to generate hydrogen, dehydrogenation of alcohols/hydrogenation of carbonyls, deoxygenation reactions, hydrogenolysis and cyclization. The input for APR is a carbohydrate solution created from plant material, and the product is a mixture of chemicals and oxygenated hydrocarbons. From there, the materials go through further conventional chemical processing to yield the final result: a mixture of non-oxygenated hydrocarbons that they claimed was cost-effective. These hydrocarbons are the exact hydrocarbons found in petroleum fuels which is why today’s cars do not need to be altered to run on biogasoline. The only difference is in origin. Petroleum based fuels are made from oil, and biogasoline is made from plants such as beets and sugarcane or cellulosic biomass which would normally be plant waste.[5]
Diesel fuel is made up of linear hydrocarbons. These are long straight carbon atom chains. They differ from the shorter, branched hydrocarbons that make up gasoline. In 2014 Researchers used a feedstock of levulinic acid to create biogasoline. Levulinic acid is derived from cellulose material, such as corn stalks, straw or other plant waste. That waste does not have to be fermented. The fuel-making process is reportedly inexpensive and offers yields of over 60 percent.[6]
Research
Research is conducted in both the academic and private sectors.
Academic
Virginia Polytechnic Institute and State University has been researching for the past four years on making stable biogasoline in current oil refineries. Their focus of the research was the length of time bio-oil’s shelf-life. The use of catalysts was used in order to remove impurities from the processed plant sugars. The researchers extended the time from three months to over a year.[7]
Iowa State University researchers use a type of fermentation in their research. They first start by forming a gaseous mixture and pyrolysize it. The result of the pyrolysis is bio-oil which the sugar rich portion is fermented and distilled to create water and ethanol. But the high acetate portion is then separated into biogasoline, water, and biomass.[8]
Private
Virent Energy Systems, Inc. which is located in Madison, Wisconsin in conjunction with Shell has developed a technique to turn plant sugars from wheat straw, corn stalks, and sugarcane pulp into biogasoline. The sugars are converted into hydrocarbons similar to those in regular gasoline by the use of catalysts.[9]
Economic viability and future
One of the major problems facing the economic viability of biogasoline is the high up- front cost. Research groups are finding that current investment groups are impatient with the pace of biogasoline progress. In addition, environmental groups may demand that biogasoline that is produced in a way that protects wildlife, especially fish.[10] A research group studying the economic viability of biofuels found that current techniques of production and high costs of production will prevent biogasoline from being accessible to the general public.[11] The group determined that the price of biogasoline would need to be approximately $800 per barrel, which they determine as unlikely with current production costs.[12] Another problem inhibiting the success of biogasoline is the lack of tax relief. The government is providing tax relief for ethanol fuels but has yet to offer tax relief for biogasoline.[13] This makes biogasoline a much less attractive option to consumers. Lastly, producing biogasoline could have a large effect on the farming industry. If biogasoline became a serious alternative, a large percentage of our existing arable land would be converted to grow crops solely for biogasoline. This could decrease the amount of land used to farm food for human consumption and may decrease overall feedstock. This would cause an increase in overall food cost.[13]
While there may be some problems facing the economic viability of biogasoline, the partnership between Royal Dutch Shell and Virent Energy Systems, Inc., a bioscience firm based in Madison, WI, to further research biogasoline is an encouraging sign for biogasoline’s future.[14] In addition, many nations are enacting policies that increase the use of biogasoline within the country to help curb the cost of fossil fuels and create more energy independence.[14] Current efforts by the partnership are focused on improving the technology and making it available for large-scale production.[14]
See also
References
- ↑ New energy act to fuel flow of 'biogasoline'
- ↑ BGT biogasoline
- ↑ "Biogasoline - Definition, Glossary, Details". Oilgea.com. Retrieved 1 December 2011.
- ↑ Ondrey, Gerald (May 2010). "This new process makes biogasoline from carbohydrates". Chemical Engineering.
- ↑ "BioForming".
|first1=missing|last1=in Authors list (help) - ↑
- ↑ DeLung, Joshua. "Turning Leftover Trees into Biogasoline". Energy.gov. United States Government. Retrieved 1 December 2011.
- ↑ "Hybrid Processing Program". Iowa State University Website. Iowa State University. Retrieved 1 December 2011.
- ↑ "Virent and Shell Start World's First Biogasoline Production Plant". Virent Energy Systems Inc. Retrieved 1 December 2011.
- ↑ Aylot, Matthew. "Forget palm oil and soya, microalgae is the next big biofuel source". The Ecologist. Retrieved 2011-11-22.
- ↑ Thom, Lindsay. "Biofuels, Bioproducts, and Biorefining". bcic.ca.
- ↑ Grimley, Chris. "Algae Case Study". Nanostring Technology.
- 1 2 Vnokurov, V. A.; A.V. Barkov; L. M. Krasnopol'skya; E.S. Mortikov (2 November 2010). "CURRENT PROBLEMS. Alternative Fuels Technology". Chemistry and Technology of Fuels and Oils. 46 (2): 75–78. doi:10.1007/s10553-010-0190-y.
- 1 2 3 Clanton, Brett. "Biogasoline: A Pile of Potential".
External links
Research institutes
- Breaking the Chemical and Engineering Barriers to Lignocellulosic Biofuels: Workshop participants list
