Biodegradation

Biodegradation is the disintegration of materials by bacteria, fungi, or other biological means.
The term is often used in relation to: biomedicine, waste management, ecology, and the bioremediation of the natural environment. It is now commonly associated with environmentally-friendly products, capable of decomposing back into natural elements.
Although often conflated, biodegradable is distinct in meaning from: compostable. While biodegradable simply means can be consumed by microorganisms, compostable makes the specific demand that the object break down under composting conditions.
Organic material can be degraded: aerobically (with oxygen) or anaerobically (without oxygen). Decomposition of biodegradable substances may include both biological and abiotic steps.
Biodegradable matter is generally organic material that provides a nutrient for microorganisms. These are so numerous and diverse that a huge range of compounds can be biodegraded, including: hydrocarbons (oils), polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs) and pharmaceutical substances. Microorganisms secrete biosurfactant, an extracellular surfactant, to enhance this process.
Factors affecting rate
In practice, almost all chemical compounds and materials are subject to biodegradation, the key is the relative rates of such processes - minutes, days, years, centuries... A number of factors determine the degradation rate of organic compounds.[2] Salient factors include light, water and oxygen. Temperature is also important because chemical reactions proceed more quickly at higher temperatures. The degradation rate of many organic compounds is limited by their bioavailability. Compounds must be released into solution before organisms can degrade them.[3]
Biodegradability can be measured in a number of ways. Respirometry tests can be used for aerobic microbes. First one places a solid waste sample in a container with microorganisms and soil, and then aerate the mixture. Over the course of several days, microorganisms digest the sample bit by bit and produce carbon dioxide – the resulting amount of CO2 serves as an indicator of degradation. Biodegradability can also be measured by anaerobic microbes and the amount of methane or alloy that they are able to produce. In formal scientific literature, the process is termed bio-remediation.[4]
| Product | Time to Biodegrade |
|---|---|
| Paper towel | 2–4 weeks |
| Newspaper | 6 weeks |
| Apple core | 2 months |
| Cardboard box | 2 months |
| Wax coated milk carton | 3 months |
| Cotton gloves | 1–5 months |
| Wool gloves | 1 year |
| Plywood | 1–3 years |
| Painted wooden sticks | 13 years |
| Plastic bags | 10–20 years |
| Tin cans | 50 years |
| Disposable diapers | 50–100 years |
| Plastic bottle | 100 years |
| Aluminium cans | 200 years |
| Glass bottles | Undetermined |
Detergents
In advanced societies, laundry detergents are based on linear alkylbenzenesulfonates. Branched alkybenzenesulfonates (below right), used in former times, were abandoned because they biodegrade too slowly.[6]
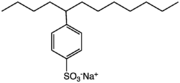 4-(5-Dodecyl) benzenesulfonate, a linear dodecylbenzenesulfonate
4-(5-Dodecyl) benzenesulfonate, a linear dodecylbenzenesulfonate A branched dodecylbenzenesulfonate, which has been phased out in developed countries.
A branched dodecylbenzenesulfonate, which has been phased out in developed countries.
Plastics
Plastics biodegrade at highly variable rates. PVC-based plumbing is specifically selected for handing sewage because PVC biodegrades very slowly. Some packaging materials on the other hand are being developed that would degrade readily upon exposure to the environment.[7] Illustrative synthetic polymers that biodegrade quickly include polycaprolactone, others polyesters and aromatic-aliphatic esters, due to their ester bonds being susceptible to attack by water. A prominent example is poly-3-hydroxybutyrate, the renewably derived polylactic acid, and the synthetic polycaprolactone. Others are the cellulose-based cellulose acetate and celluloid (cellulose nitrate).
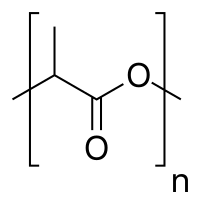 Polylactic acid is an example of a plastic that biodegrades quickly.
Polylactic acid is an example of a plastic that biodegrades quickly.
Under low oxygen conditions biodegradable plastics break down slower and with the production of methane, like other organic materials do. The breakdown process is accelerated in a dedicated compost heap. Starch-based plastics will degrade within two to four months in a home compost bin, while polylactic acid is largely undecomposed, requiring higher temperatures.[8] Polycaprolactone and polycaprolactone-starch composites decompose slower, but the starch content accelerates decomposition by leaving behind a porous, high surface area polycaprolactone. Nevertheless, it takes many months.[9] In 2016, a bacterium named Ideonella sakaiensis was found to biodegrade PET.
Many plastic producers have gone so far even to say that their plastics are compostable, typically listing corn starch as an ingredient. However, these claims are questionable because the plastics industry operates under its own definition of compostable:
- "that which is capable of undergoing biological decomposition in a compost site such that the material is not visually distinguishable and breaks down into carbon dioxide, water, inorganic compounds and biomass at a rate consistent with known compostable materials." (Ref: ASTM D 6002)[10]
The term "composting" is often used informally to describe the biodegradation of packaging materials. Legal definitions exist for compostability, the process that leads to compost. Four criteria are offered by the European Union:[11][12]
- Chemical composition: volatile matter and heavy metals as well as fluorine should be limited.
- Biodegradability: the conversion of >90% of the original material into CO2, water and minerals by biological processes within 6 months.
- Disintegrability: at least 90% of the original mass should be decomposed into particles that are able to pass through a 2x2 mm sieve.
- Quality: absence of toxic substances and other substances that impede composting.
Biodegradable technology
In 1973 it was proven for the first time that polyester degrades when disposed in bioactive material such as soil. Polyesters are water resistant and can be melted and shaped into sheets, bottles, and other products, making certain plastics now available as a biodegradable product. Following, polyhydroxylalkanoates (PHAs) were produced directly from renewable resources by microbes. They are approximately 95% cellular bacteria and can be manipulated by genetic strategies. The composition and biodegradability of PHAs can be regulated by blending it with other natural polymers. In the 1980s the company ICI Zenecca commercialized PHAs under the name Biopol. It was used for the production of shampoo bottles and other cosmetic products. Consumer response was unusual. Consumers were willing to pay more for this product because it was natural and biodegradable, which had not occurred before.[13]
Now biodegradable technology is a highly developed market with applications in product packaging, production and medicine. Biodegradable technology is concerned with the manufacturing science of biodegradable materials. It imposes science-based mechanisms of plant genetics into the processes of today. Scientists and manufacturing corporations can help impact climate change by developing a use of plant genetics that would mimic some technologies. By looking to plants, such as biodegradable material harvested through photosynthesis, waste and toxins can be minimized.[14]
Oxo-biodegradable technology, which has further developed biodegradable plastics, has also emerged. Oxo-biodegradation is defined by CEN (the European Standards Organisation) as "degradation resulting from oxidative and cell-mediated phenomena, either simultaneously or successively." Whilst sometimes described as "oxo-fragmentable," and "oxo-degradable" this describes only the first or oxidative phase. These descriptions should not be used for material which degrades by the process of oxo-biodegradation defined by CEN, and the correct description is "oxo-biodegradable."
By combining plastic products with very large polymer molecules, which contain only carbon and hydrogen, with oxygen in the air, the product is rendered capable of decomposing in anywhere from a week to one to two years. This reaction occurs even without prodegradant additives but at a very slow rate. That is why conventional plastics, when discarded, persist for a long time in the environment. Oxo-biodegradable formulations catalyze and accelerate the biodegradation process but it takes considerable skill and experience to balance the ingredients within the formulations so as to provide the product with a useful life for a set period, followed by degradation and biodegradation.[15]
Biodegradable technology is especially utilized by the bio-medical community. Biodegradable polymers are classified into three groups: medical, ecological, and dual application, while in terms of origin they are divided into two groups: natural and synthetic.[16] The Clean Technology Group is exploiting the use of supercritical carbon dioxide, which under high pressure at room temperature is a solvent that can use biodegradable plastics to make polymer drug coatings. The polymer (meaning a material composed of molecules with repeating structural units that form a long chain) is used to encapsulate a drug prior to injection in the body and is based on lactic acid, a compound normally produced in the body, and is thus able to be excreted naturally. The coating is designed for controlled release over a period of time, reducing the number of injections required and maximizing the therapeutic benefit. Professor Steve Howdle states that biodegradable polymers are particularly attractive for use in drug delivery, as once introduced into the body they require no retrieval or further manipulation and are degraded into soluble, non-toxic by-products. Different polymers degrade at different rates within the body and therefore polymer selection can be tailored to achieve desired release rates.[17]
Other biomedical applications include the use of biodegradable, elastic shape-memory polymers. Biodegradable implant materials can now be used for minimally invasive surgical procedures through degradable thermoplastic polymers. These polymers are now able to change their shape with increase of temperature, causing shape memory capabilities as well as easily degradable sutures. As a result, implants can now fit through small incisions, doctors can easily perform complex deformations, and sutures and other material aides can naturally biodegrade after a completed surgery.[18]
Etymology of "biodegradable"
The first known use of the word in biological text was in 1961 when employed to describe the breakdown of material into the base components of carbon, hydrogen, and oxygen by microorganisms. Now biodegradable is commonly associated with environmentally friendly products that are part of the earth's innate cycle and capable of decomposing back into natural elements.
See also
- Anaerobic digestion
- Biodegradability prediction
- Biodegradable electronics
- Biodegradable polythene film
- Biodegradation (journal)
- Bioplastic – biodegradable, bio-based plastics
- Bioremediation
- Decomposition – reduction of the body of a formerly living organism into simpler forms of matter
- Landfill gas monitoring
- List of environment topics
- Microbial biodegradation
- Photodegradation
References
- ↑ "Terminology for biorelated polymers and applications (IUPAC Recommendations 2012)" (PDF). Pure and Applied Chemistry. 84 (2): 377–410. 2012. doi:10.1351/PAC-REC-10-12-04.
- ↑ Sims, G. K. and A.M. Cupples. 1999. Factors controlling degradation of pesticides in soil. Pesticide Science 55:598–601.
- ↑ Sims, G.K. (1991). The effects of sorption on the bioavailability of pesticides. London: Springer Verlag. pp. 119–137.
- ↑ "Measuring Biodegradability", The University of Waikato, June 19, 2008
- ↑ "Marine Debris Biodegradation Time Line". C-MORE, citing Mote Marine Laboratory, 1993.
- ↑ Kurt Kosswig,"Surfactants" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, 2005, Weinheim. doi:10.1002/14356007.a25_747
- ↑ Kyrikou, Ioanna; Briassoulis, Demetres (12 Apr 2007). "Biodegradation of Agricultural Plastic Films: A Critical Review". Journal of Polymers and the Environment. SpringerLink . 15 (2): 125–150. doi:10.1007/s10924-007-0053-8. Retrieved 30 May 2015.
- ↑ "Microsoft Word - SECTION 6 BIODEGRADABILITY OF PACKAGING WASTE.doc" (PDF). Www3.imperial.ac.uk. Retrieved 2014-03-02.
- ↑ Fig.9
- ↑ "Compostable.info". Compostable.info. Retrieved 2014-03-02.
- ↑ "Requirements of the EN 13432 standard" (PDF). www.okcompost.be. Brussels, Belgium: Vinçotte. Retrieved July 22, 2017.
- ↑ M. Breulmann et al. "Polymers, Biodegradable" in Ullmann's Encyclopedia of Industrial Chemistry 2012 Wiley-VCH, Weinheim.doi:10.1002/14356007.n21_n01
- ↑ Gross,Richard. "Biodegradable Polymers for the Environment", American Association of Advanced Science, August 2, 2002, p. 804.
- ↑ Luzier, W. D. "Materials Derived from Biomass/Biodegradable Materials." Proceedings of the National Academy of Sciences 89.3 (1992): 839–42. Print.
- ↑ Agamuthu, P."Biodegradability and Degradability of Plastic Waste", "International Solid Waste Association" November 9, 2004
- ↑ Yoshito, Ikada. "Biodegradable Polyesters for Medical and Ecological Applications", "Massachusetts Institute of Technology", 2000. p117
- ↑ "Using Green Chemistry to Deliver Cutting Edge Drugs". The University of Nottingham. September 13, 2007.
- ↑ Lendlein, Andreas. "Biodegradable, Elastic Shape-Memory Polymers for Potential Biomedical Applications". American Association of Advancement of Science, 2002, p 1673.
Standards by ASTM International
- D5210- Standard Test Method for Determining the Anaerobic Biodegradation of Plastic Materials in the Presence of Municipal Sewage Sludge
- D5526- Standard Test Method for Determining Anaerobic Biodegradation of Plastic Materials Under Accelerated Landfill Conditions
- D5338- Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials Under Controlled Composting Conditions, Incorporating Thermophilic Temperatures
- D5511- Standard Test Method for Determining Anaerobic Biodegradation of Plastic Materials Under High-Solids Anaerobic-Digestion Conditions
- D5864- Standard Test Method for Determining Aerobic Aquatic Biodegradation of Lubricants or Their Components
- D5988- Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials in Soil
- D6139- Standard Test Method for Determining the Aerobic Aquatic Biodegradation of Lubricants or Their Components Using the Gledhill Shake Flask
- D6006- Standard Guide for Assessing Biodegradability of Hydraulic Fluids
- D6340- Standard Test Methods for Determining Aerobic Biodegradation of Radiolabeled Plastic Materials in an Aqueous or Compost Environment
- D6691- Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials in the Marine Environment by a Defined Microbial Consortium or Natural Sea Water Inoculum
- D6731-Standard Test Method for Determining the Aerobic, Aquatic Biodegradability of Lubricants or Lubricant Components in a Closed Respirometer
- D6954- Standard Guide for Exposing and Testing Plastics that Degrade in the Environment by a Combination of Oxidation and Biodegradation
- D7044- Standard Specification for Biodegradable Fire Resistant Hydraulic Fluids
- D7373-Standard Test Method for Predicting Biodegradability of Lubricants Using a Bio-kinetic Model
- D7475- Standard Test Method for Determining the Aerobic Degradation and Anaerobic Biodegradation of Plastic Materials under Accelerated Bioreactor Landfill Conditions
- D7665- Standard Guide for Evaluation of Biodegradable Heat Transfer Fluids
External links
- European Bioplastics Association
- The Science of Biodegradable Plastics: The Reality Behind Biodegradable Plastic Packaging Material
- Biodegradable Polyesters for Medical and Ecological Applications
- Biodegradable Plastic Definition