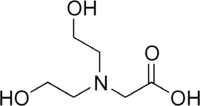
Bicine
 | |
| Names | |
|---|---|
| IUPAC name
2-(Bis(2-hydroxyethyl)amino)acetic acid | |
| Other names
N,N-Bis(2-hydroxyethyl)glycine; Diethylolglycine; Diethanol glycine; Dihydroxyethylglycine; BHG | |
| Identifiers | |
| 3D model (JSmol) |
|
| Abbreviations | DHEG |
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.005.233 |
| PubChem CID |
|
| |
| |
| Properties | |
| C6H13NO4 | |
| Molar mass | 163.17 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Bicine is an organic compound used as a buffering agent. It is one of Good's buffers and has a pKa of 8.35 at 20 °C.[1] It is prepared by the reaction of glycine with ethylene oxide, followed by hydrolysis of the resultant lactone.[2]
Bicine is a contaminant in amine systems used for gas sweetening. It is formed by amine degradation in the presence of O2, SO2, H2S or Thiosulfate.[3]
See also
References
- ↑ N,N-Bis(2-hydroxyethyl)glycine at ChEBI
- ↑ The Merck Index (10th ed.). Rahway, NJ: Merck & Co. 1983. p. 453. ISBN 0-911910-27-1.
- ↑ Lawson, Gary (2003). "Amine Plant Corrosion Reduced by Removal of Bicine" (PDF). Gas Processors Association Annual Convention. Retrieved March 2016. Check date values in:
|access-date=(help)
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.