Beta thalassemia
| Beta thalassemia | |
|---|---|
 | |
| Beta thalassemia genetics | |
| Classification and external resources | |
| Specialty | Hematology |
| ICD-10 | D56.1 |
| ICD-9-CM | 282.44 |
| OMIM | 141900 |
| DiseasesDB | 3087 1373 |
| eMedicine | article/199534 |
| MeSH | D017086 |
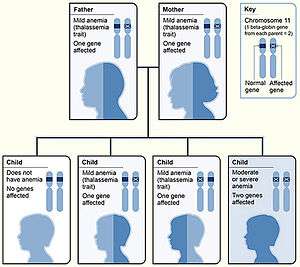
Beta thalassemias (β thalassemias) are a group of inherited blood disorders. They are forms of thalassemia caused by reduced or absent synthesis of the beta chains of hemoglobin that result in variable outcomes ranging from severe anemia to clinically asymptomatic individuals. Global annual incidence is estimated at one in 100,000.[1] Beta thalassemias are caused by mutations in the HBB gene on chromosome 11, inherited in an autosomal recessive fashion. The severity of the disease depends on the nature of the mutation.[2]
HBB blockage over time leads to decreased beta-chain synthesis. The body's inability to construct new beta-chains leads to the underproduction of HbA.[3] Reductions in HbA available overall to fill the red blood cells in turn leads to microcytic anemia. Microcytic anemia ultimately develops in respect to inadequate HBB protein for sufficient red blood cell functioning.[4] Due to this factor, the patient may require blood transfusions to make up for the blockage in the beta-chains. Repeated blood transfusions can lead to build-up of iron overload, ultimately resulting in iron toxicity. This iron toxicity can cause various problems, including myocardial siderosis and heart failure leading to the patient’s death.[5]
Signs and symptoms
Three main forms have been described: thalassemia major, thalassemia intermedia, and thalassemia minor. All people with thalassemia are susceptible to health complications that involve the spleen (which is often enlarged and frequently removed) and gallstones.[6] These complications are mostly found in thalassemia major and intermedia patients. Individuals with beta thalassemia major usually present within the first two years of life with severe anemia, poor growth, and skeletal abnormalities during infancy. Untreated thalassemia major eventually leads to death, usually by heart failure; therefore, birth screening is very important.[7]
Excess iron causes serious complications within the liver, heart, and endocrine glands. Severe symptoms include liver cirrhosis, liver fibrosis, and in extreme cases, liver cancer.[8] Heart failure, growth impairment, diabetes and osteoporosis are life-threatening contributors brought upon by TM.[9] The main cardiac abnormalities seen to have resulted from thalassemia and iron overload include left ventricular systolic and diastolic dysfunction, pulmonary hypertension, valveulopathies, arrhythmias, and pericarditis. Increased gastrointestinal iron absorption is seen in all grades of beta thalassemia and increased red blood cell destruction by the spleen due to ineffective erythropoiesis further releases additional iron into the bloodstream.[10]
Cause
Mutations
Two major groups of mutations can be distinguished:
- Nondeletion forms: These defects, in general, involve a single base substitution or small insertions near or upstream of the β globin gene. Most often, mutations occur in the promoter regions preceding the beta-globin genes. Less often, abnormal splice variants are believed to contribute to the disease.[11]
- Deletion forms: Deletions of different sizes involving the β globin gene produce different syndromes such as (βo) or hereditary persistence of fetal hemoglobin syndromes.[12]
| Name | Older synonyms | Description | Alleles |
|---|---|---|---|
| Thalassemia minor | Heterozygous form: Only one of β globin alleles bears a mutation. Individuals will suffer from microcytic anemia. Detection usually involves lower than normal mean corpuscular volume value (<80 fL).[13] | β+/β βo/β | |
| Thalassemia intermedia | Affected individuals can often manage a normal life but may need occasional transfusions, e.g., at times of illness or pregnancy, depending on the severity of their anemia.[14] | β+/β+ βo/β+ | |
| Thalassemia major | Mediterranean anemia; Cooley anemia | Homozygous form: Occurs when both alleles have thalassemia mutations. This is a severe microcytic, hypochromic anemia. Untreated, it causes anemia, splenomegaly and severe bone deformities. It progresses to death before age 20. Treatment consists of periodic blood transfusion; splenectomy for splenomegaly and chelation of transfusion-related iron overload.[15] | βo/βo |
mRNA assembly

Beta thalassemia is a hereditary disease affecting hemoglobin. As with about half of all hereditary diseases,[16] an inherited mutation damages the assembly of the messenger-type RNA (mRNA) that is transcribed from a chromosome. DNA contains both the instructions (genes) for stringing amino acids together into proteins, as well as stretches of DNA that play important roles in regulating produced protein levels.[17]
In thalassemia, an additional, contiguous length or a discontinuous fragment of non-coding instructions are included in the mRNA. This happens because the mutation obliterates the boundary between the intronic and exonic portions.[18] Because all the coding sections may still be present, normal hemoglobin may be produced and the added material, if it produces pathology, instead disrupts regulatory functions enough to produce anemia.Hemoblogin's normal alpha and beta subunits each have an iron-containing central portion (heme) that allows the protein chain of a subunit to fold around it. Normal adult hemoglobin contains 2 alpha and 2 beta subunits.[19] Thalassemias typically affect only the mRNAs for production of the beta chains (hence the name). Since the mutation may be a change in only a single base (a "Single Nucleotide Polymorphism"), on-going efforts seek gene therapies to make that single correction.[20][21]
Risk Factors
Family history and ancestry are factors that increase the risk of beta thalassemia. Depending on your family history, if your parents or grandparents suffered from beta thalassemia there is a high probability of the mutated gene being inherited by an offspring. Even if a child does not have beta thalassemia major, they can still be a carrier resulting in future offspring having beta thalassemia. Another risk factor is because of certain ancestry. Beta thalassemia occurs most often in people of Italian, Greek, Middle Eastern, Southern Asian, and African ancestry.[22]
Diagnosis
Abdominal pain due to hypersplenism and splenic infarction and right-upper quadrant pain caused by gallstones are major clinical manifestations. However, diagnosing thalassemiæ from symptoms alone is inadequate. Physicians note these signs as associative due to this disease's complexity.[23] The following associative signs can attest to the severity of the phenotype: pallor, poor growth, inadequate food intake, splenomegaly, jaundice, maxillary hyperplasia, dental malocclusion, cholelithiasis, systolic ejection murmur in the presence of severe anemia and pathologic fractures. Based on symptoms, tests are ordered for a differential diagnosis. These tests include complete blood count; hemoglobin electrophoresis; serum transferrin, ferritin, total iron-binding capacity; urine urobilin and urobilogen; peripheral blood smear, which may show codocytes, or target cells;[24] hematocrit; and serum bilirubin.[25][26]
DNA analysis
All beta thalassemias may exhibit abnormal red blood cells, a family history is followed by DNA analysis.[27] This test is used to investigate deletions and mutations in the alpha- and beta-globin-producing genes. Family studies can be done to evaluate carrier status and the types of mutations present in other family members. DNA testing is not routine, but can help diagnose thalassemia and determine carrier status. In most cases the treating physician uses a clinical prediagnosis assessing anemia symptoms: fatigue, breathlessness and poor exercise tolerance.[28] Further genetic analysis may include HPLC should routine electrophoresis prove difficult.[25]
Prevention
Beta thalassemia is a hereditary disease allowing for a preventative treatment by carrier screening and prenatal diagnosis. It can be prevented if one parent has normal genes, giving rise to screenings that empower carriers to select partners with normal hemoglobin. A study aimed at detecting the genes that could give rise to offspring with sickle cell disease. Patients diagnosed with beta thalassemia have MCH ≤ 26 pg and an RDW < 19. Of 10,148 patients, 1,739 patients had a hemoglobin phenotype and RDW consistent with beta thalassemia. After the narrowing of patients, the HbA2 levels were tested presenting 77 patients with beta thalassemia.[29] This screening procedure proved insensitive in populations of West African ancestry because of the indicators has high prevalence of alpha thalassemia. Countries have programs distributing information about the reproductive risks associated with carriers of haemoglobinopathies. Thalassemia carrier screening programs have educational programs in schools, armed forces, and through mass media as well as providing counseling to carriers and carrier couples.[30] Screening has showed reduced incidence; by 1995 the prevalence in Italy reduced from 1:250 to 1:4000, and a 95% decrease in that region. The decrease in incidence has benefitted those affected with thalassemia, as the demand for blood has decreased, therefore improving the supply of treatment.
Treatment
Beta thalassemia major
Affected children require regular lifelong blood transfusion and can have complications, which may involve the spleen. Bone marrow transplants can be curative for some children.[31] Patients receive frequent blood transfusions that lead to or potentiate iron overload.[32] Iron chelation treatment is necessary to prevent damage to internal organs. Advances in iron chelation treatments allow patients with thalassemia major to live long lives with access to proper treatment. Popular chelators include deferoxamine and deferiprone.[33][34]
The most common patient deferoxamine complaint is that they are painful and inconvenient. The oral chelator deferasirox was approved for use in 2005 in some countries,[35][36] it offers some hope with compliance at a higher cost. Bone marrow transplantation is the only cure and is indicated for patients with severe thalassemia major. Transplantation can eliminate a patient's dependence on transfusions. Absent a matching donor, a savior sibling can be conceived by preimplantation genetic diagnosis (PGD) to be free of the disease as well as to match the recipient's human leukocyte antigen (HLA) type.[37]
Scientists at Weill Cornell Medical College have developed a gene therapy strategy that could feasibly treat both beta-thalassemia and sickle cell disease. The technology is based on delivery of a lentiviral vector carrying both the human β-globin gene and an ankyrin insulator to improve gene transcription and translation, and boost levels of β-globin production.[38]
Surgical
Patients with thalassemia major are more inclined to have a splenectomy. The medical cases of splenectomies have been declining in recent years due to decreased prevalence of hypersplenism in adequately transfused patients. Patients with hypersplenism are inclined to have a lower amount of healthy blood cells in their body than normal and reveal symptoms of anemia. Iron rich patients need a splenectomy to reduce the probability of an iron overload. The different surgical techniques are the open and laparoscopic method.[39] The laparoscopic method requires longer operating time but a shorter recovery period with no surgical scar. If it is unnecessary to remove the entire spleen a partial splenectomy may occur; this method preserves some of the immune function while reducing the probability of hypersplenism. Surgeons who chose Laparoscopic splenectomy must administer an appropriate immunization at least two weeks before the surgery. On the operating table the patient must be placed at a 30˚ to 40˚ position with his or her left arm elevated above the head to properly make the incision. The camera is inserted along with four other trocars: one placed in the left subcostal area, one inserted at the midpoint between the first and third, one 4 cm right of the midline, and the fourth positioned on the midline to retract the spleen.[40] Removing the spleen, an organ in under a person’s rib cage on the upper left side of its abdominal, allows the body to regulate the correct amount of healthy cells in the body.
Therapeutic
Long-term transfusion therapy to maintain the patient’s hemoglobin level above 9-10 g/dL (normal levels are 13.8 for males, and 12.1 for females). Patients are transfused by meeting strict criteria ensuring their safety. They must have: confirmed laboratory diagnosis of thalassemia major, and hemoglobin levels less than 7g/dL, to be eligible for the transfusion. To ensure quality blood transfusions, the packed red blood cells should be leucoreduced with a minimum of 40g of hemoglobin content. By having leucoreduced blood packets, the patient is at a lower risk to develop adverse reactions by contaminated white cells and preventing platelet alloimmunisation.[41] Pre-storage filtration of whole blood offers high efficiency for removal and low residual of leukocytes; It is the preferred method of leucoreduction compared to pre-transfusion and bedside filtration. Patients with allergic transfusion reactions or unusual red cell antibodies must received “washed red cells” or “cryopreserved red cells.” Washed red cells have been removed of plasma proteins that would have become a target of the patient’s antibodies allowing the transfusion to be carried out safely. Cryopreserved red cells are used to maintain a supply of rare donor units for patients with unusual red cell antibodies or missing common red cell antigens. The transfusion programs available involve lifelong regular blood transfusion to main the pre-transfusion hemoglobin level above 9-10 g/gL.[39] The monthly transfusions promote normal growth, physical activities, suppress bone marrow activity, and minimize iron accumulation.
Pharmaceutical
Iron overload is an unavoidable consequence of chronic transfusion therapy, necessary for patients with beta thalassemia. Iron chelation is a medical therapy that avoids the complications of iron overload. The iron overload can be removed by Deferasirox, an oral iron chelator, which has a dose- dependent effect on iron burden. Every unit of transfused blood contains 200–250 mg of iron and the body has no natural mechanism to remove excess iron Deferasirox is a vital part in the patients health after blood transfusions.[42] During normal iron homeostasis the circulating iron is bound to transferrin, but with an iron overload, the ability for transferrin to bind iron is exceeded and non-transferrin bound iron is formed. It represents a potentially toxic iron form due to its high propensity to induce oxygen species and is responsible for cellular damage. The prevention of iron overload protects patients from morbidity and mortality. The primary aim is to bind to and remove iron from the body and a rate equal to the rate of transfusional iron input or greater than iron input.[43] During clinical trails patients that received Deferasirox experienced no drug-related neutropenia or agranulocytosis, which was present with other iron chelators. Its long half life requires it to be taken once daily and provides constant chelation. Cardiac failure is a main cause of illness from transfusional iron overload but deferasirox demonstrated the ability to remove iron from iron-loaded myocardial cells protecting beta thalassemia patients from effects of required blood transfusions.
Beta thalassemia intermedia
Patients may require episodic blood transfusions. Transfusion-dependent patients develop iron overload and require chelation therapy[44] to remove the excess iron. Transmission is autosomal recessive; however, dominant mutations and compound heterozygotes have been reported. Genetic counseling is recommended and prenatal diagnosis may be offered.[45] Alleles without a mutation that reduces function are characterized as (β). Mutations are characterized as (βo) if they prevent any formation of β chains,[46] mutations are characterized as (β+) if they allow some β chain formation to occur.
Beta thalassemia minor
Patients are often monitored without treatment. While many of those with minor status do not require transfusion therapy, they still risk iron overload, particularly in the liver. A serum ferritin test checks iron levels and can point to further treatment.[47] Although not life-threatening on its own, it can affect quality of life due to the anemia. Minor often coexists with other conditions such as asthma and can cause iron overload of the liver and in those with non-alcoholic fatty liver disease, lead to more severe outcomes.[48]
Epidemiology
The beta form of thalassemia is particularly prevalent among the Mediterranean peoples and this geographical association is responsible for its naming: thalassa (θάλασσα) is the Greek word for sea and haema (αἷμα) is the Greek word for blood. In Europe, the highest concentrations of the disease are found in Greece and the Turkish coastal regions. The major Mediterranean islands (except the Balearics) such as Sicily, Sardinia, Corsica, Cyprus, Malta and Crete are heavily affected in particular.[49][50] Other Mediterranean peoples, as well as those in the vicinity of the Mediterranean, also have high incidence rates, including people from West Asia and North Africa. The data indicate that 15% of the Greek and Turkish Cypriots are carriers of beta-thalassaemia genes, while 10% of the population carry alpha-thalassaemia genes.[51]
Evolutionary adaptation
The thalassemia trait may confer a degree of protection against malaria,[52] which is or was prevalent in the regions where the trait is common, thus conferring a selective survival advantage on carriers (known as heterozygous advantage), thus perpetuating the mutation. In that respect, the various thalassemias resemble another genetic disorder affecting hemoglobin, sickle-cell disease.[53]
Incidence
The disorder affects all genders but is more prevalent in certain ethnicities and age groups. 20 people die per year causing thalassemia to be listed as a “rare disease”. In the United States, thalassemia’s prevalence is approximately 1 in 272,000 or 1,000 people. There have been 4,000 hospitalized cases in England in 2002 and 9,233 consultant episodes for thalassemia. Men accounted for 53% of hospital consultant episodes and women accounted for 47%. The mean patient age is 23 with only 1% of consultants the patient is older than 75 and 69% were 15-59 year olds. The Children’s Hospital Oakland formed an international network to combat thalassemia.[54] “It is the world’s most common genetic blood disorder and is rapidly increasing”. 7% of the world’s population are carriers and 400,000 babies are born with the trait annually. It is usually fatal in infancy if blood transfusion are not initiated immediately.[55]
See also
References
- ↑ Galanello, Renzo; Origa, Raffaella (21 May 2010). "Beta-thalassemia". Orphanet J Rare Dis. Orphanet Journal of Rare Diseases. 5: 11. PMC 2893117
 . PMID 20492708. doi:10.1186/1750-1172-5-11.
. PMID 20492708. doi:10.1186/1750-1172-5-11. - ↑ Goldman, Lee; Schafer, Andrew I. (2015-04-21). Goldman-Cecil Medicine: Expert Consult - Online. Elsevier Health Sciences. ISBN 9780323322850.
- ↑ Carton, James (2012-02-16). Oxford Handbook of Clinical Pathology. OUP Oxford. ISBN 9780191629938.
- ↑ Perkin, Ronald M.; Newton, Dale A.; Swift, James D. (2008). Pediatric Hospital Medicine: Textbook of Inpatient Management. Lippincott Williams & Wilkins. ISBN 9780781770323.
- ↑ Galanello, Renzo; Origa, Raffaella (2010-05-21). "Beta-thalassemia". Orphanet Journal of Rare Diseases. 5 (1): 11. ISSN 1750-1172. PMC 2893117
 . PMID 20492708. doi:10.1186/1750-1172-5-11.
. PMID 20492708. doi:10.1186/1750-1172-5-11. - ↑ "Beta thalassemia". Genetics Home Reference. Retrieved 2015-05-26.
- ↑ Introduction to Pathology for the Physical Therapist Assistant. Jones & Bartlett Publishers. 2011. ISBN 9780763799083.
- ↑ Anderson, Gregory J.; McLaren, Gordon D. (2012-01-16). Iron Physiology and Pathophysiology in Humans. Springer Science & Business Media. ISBN 9781603274845.
- ↑ Barton, James C.; Edwards, Corwin Q.; Phatak, Pradyumna D.; Britton, Robert S.; Bacon, Bruce R. (2010-07-22). Handbook of Iron Overload Disorders. Cambridge University Press. ISBN 9781139489393.
- ↑ McCance, Kathryn L.; Huether, Sue E. (2013-12-13). Pathophysiology: The Biologic Basis for Disease in Adults and Children. Elsevier Health Sciences. ISBN 9780323088541.
- ↑ Leonard, Debra G. B. (2007-11-25). Molecular Pathology in Clinical Practice. Springer Science & Business Media. ISBN 9780387332277.
- ↑ Bowen, Juan M.; Mazzaferri, Ernest L. (2012-12-06). Contemporary Internal Medicine: Clinical Case Studies. Springer Science & Business Media. ISBN 9781461567134.
- ↑ Disorders, National Organization for Rare (2003). NORD Guide to Rare Disorders. Lippincott Williams & Wilkins. ISBN 9780781730631.
- ↑ Barton, James C.; Edwards, Corwin Q. (2000-01-13). Hemochromatosis: Genetics, Pathophysiology, Diagnosis and Treatment. Cambridge University Press. ISBN 9780521593809.
- ↑ Wilkins, Lippincott Williams & (2009). Professional Guide to Diseases. Lippincott Williams & Wilkins. ISBN 9780781778992.
- ↑ Ward, Amanda J; Cooper, Thomas A (2009). "The pathobiology of splicing". The Journal of Pathology. 220 (2): 152–63. PMC 2855871
 . PMID 19918805. doi:10.1002/path.2649.
. PMID 19918805. doi:10.1002/path.2649. - ↑ "the definition of dna". Dictionary.com. Retrieved 2015-05-26.
- ↑ Okpala, Iheanyi (2008-04-15). Practical Management of Haemoglobinopathies. John Wiley & Sons. ISBN 9781405140201.
- ↑ Vasudevan, D. M.; Sreekumari, S.; Vaidyanathan, Kannan (2011-11-01). Textbook of Biochemistry for Dental Students. JP Medical Ltd. ISBN 9789350254882.
- ↑ Taeusch, H. William; Ballard, Roberta A.; Gleason, Christine A.; Avery, Mary Ellen (2005). Avery's Diseases of the Newborn. Elsevier Health Sciences. ISBN 0721693474.
- ↑ Beta Thalassemia: New Insights for the Healthcare Professional: 2013 Edition: ScholarlyBrief. ScholarlyEditions. 2013-07-22. ISBN 9781481663472.
- ↑ "Risk Factors". Mayo Clinic. Retrieved 4 April 2017.
- ↑ "How Are Thalassemias Diagnosed? - NHLBI, NIH". www.nhlbi.nih.gov. Retrieved 2015-05-26.
- ↑ Target Cells, Imperial College of London Department of Medicine
- 1 2 Orkin, Stuart H.; Nathan, David G.; Ginsburg, David; Look, A. Thomas; Fisher, David E.; Lux, Samuel (2009). Nathan and Oski's Hematology of Infancy and Childhood (7th ed.). Philadelphia: Saunders. ISBN 978-1-4160-3430-8.
- ↑ "What Are the Signs and Symptoms of Thalassemias? - NHLBI, NIH". www.nhlbi.nih.gov. Retrieved 2015-05-26.
- ↑ McKinney, Emily Slone; James, Susan R.; Murray, Sharon Smith; Nelson, Kristine; Ashwill, Jean (2014-04-17). Maternal-Child Nursing. Elsevier Health Sciences. ISBN 9780323293778.
- ↑ Schrijver, Iris (2011-09-09). Diagnostic Molecular Pathology in Practice: A Case-Based Approach. Springer Science & Business Media. ISBN 9783642196775.
- ↑ Cousens, N. E.; Gaff, C. L.; Metcalfe, S. A.; Delatycki, M. B. (2010). "Carrier screening for Beta-thalassaemia: a review of international practice". European Journal of Human Genetics. 18 (10): 1077–83. PMC 2987452
 . PMID 20571509. doi:10.1038/ejhg.2010.90.
. PMID 20571509. doi:10.1038/ejhg.2010.90. - ↑ "Screening for the beta-thalassaemia trait: hazards among populations of West African Ancestry". Retrieved 4 April 2017.
- ↑ Muncie, Herbert L.; Campbell, James S. (2009). "Alpha and Beta Thalassemia". American Family Physician. 80 (4): 339–44. PMID 19678601.
- ↑ Greer, John P.; Arber, Daniel A.; Glader, Bertil; List, Alan F.; Means, Robert T.; Paraskevas, Frixos; Rodgers, George M. (2013-08-29). Wintrobe's Clinical Hematology. Lippincott Williams & Wilkins. ISBN 9781469846224.
- ↑ Greer, John P.; Arber, Daniel A.; Glader, Bertil; List, Alan F.; Means, Robert T.; Paraskevas, Frixos; Rodgers, George M. (2013-08-29). Wintrobe's Clinical Hematology. Lippincott Williams & Wilkins. ISBN 9781469846224.
- ↑ Hydroxamic Acids: Advances in Research and Application: 2011 Edition: ScholarlyPaper. ScholarlyEditions. 2012-01-09. ISBN 9781464952081.
- ↑ "NCBI - WWW Error Blocked Diagnostic". pubchem.ncbi.nlm.nih.gov. Retrieved 2015-05-26.
- ↑ "Deferoxamine". livertox.nih.gov. Retrieved 2015-05-26.
- ↑ Sabloff, Mitchell; Chandy, Mammen; Wang, Zhiwei; Logan, Brent R.; Ghavamzadeh, Ardeshir; Li, Chi-Kong; Irfan, Syed Mohammad; Bredeson, Christopher N.; Cowan, Morton J. (2011). "HLA-matched sibling bone marrow transplantation for β-thalassemia major". Blood. 117 (5): 1745–1750. ISSN 0006-4971. PMC 3056598
 . PMID 21119108. doi:10.1182/blood-2010-09-306829.
. PMID 21119108. doi:10.1182/blood-2010-09-306829. - ↑ "Gene Therapy Shows Promise for Treating Beta-Thalassemia and Sickle Cell Disease". Retrieved 2015-10-15.
- 1 2 Advani, Pooja. "Beta Thalassemia Treatment & Management". Medscape. Retrieved 4 April 2017.
- ↑ Uranüs, Selman. "Splenectomy for hematological disorders". NCBI. Retrieved 4 April 2017.
- ↑ A, Cohen. "Blood Transfusion Therapy in β-Thalassaemia Major". NCBI. Retrieved 4 April 2017.
- ↑ Cappellini, Maria. "Exjade® (deferasirox, ICL670) in the treatment of chronic iron overload associated with blood transfusion". PMC 1936310
 .
. - ↑ Advani, Pooja. "Beta Thalassemia Medication". Medscape. Retrieved 4 April 2017.
- ↑ Schwartz, M. William (2012). The 5 Minute Pediatric Consult. Lippincott Williams & Wilkins. ISBN 9781451116564.
- ↑ Porwit, Anna; McCullough, Jeffrey; Erber, Wendy N. (2011-05-27). Blood and Bone Marrow Pathology. Elsevier Health Sciences. ISBN 0702045357.
- ↑ Hemoglobinopathies. Jaypee Brothers Publishers. 2006. ISBN 9788180616693.
- ↑ Torre, Dario M.; Lamb, Geoffrey C.; Ruiswyk, Jerome Van; Schapira, Ralph M. (2009). Kochar's Clinical Medicine for Students. Lippincott Williams & Wilkins. ISBN 9780781766999.
- ↑ Brissot, Pierre; Cappellini, Maria Domenica (2014). "LIVER DISEASE".
- ↑ "WHO | Global epidemiology of haemoglobin disorders and derived service indicators". www.who.int. Retrieved 2015-05-26.
- ↑ Berg, Sheri; Bittner, Edward A. (2013-10-16). The MGH Review of Critical Care Medicine. Lippincott Williams & Wilkins. ISBN 9781451173680.
- ↑ Haematology Made Easy. AuthorHouse. 2013-02-06. ISBN 9781477246511.
- ↑ Abouelmagd, Ahmed; Ageely, Hussein M. (2013). Basic Genetics: A Primer Covering Molecular Composition of Genetic Material, Gene Expression and Genetic Engineering, and Mutations and Human Genetic. Universal-Publishers. ISBN 9781612331928.
- ↑ Weatherall, David J. Lichtman, MA,; Kipps, TJ,; Seligsohn, U,; Kaushansky, K,; Prchal, JT, eds. The Thalassemias: Disorders of Globin Synthesis. Williams Hematology (8 ed.).
- ↑ "Statistics about Thalassemia". Right Diagnosis. Retrieved 4 April 2017.
- ↑ "Thalassemia: Genetic Blood Disorder Expected To Double In Life History of a Dinosaur: M... Next FeBlwue WDhaelecs aOpdtimeisze". ScienceDaily. Retrieved 4 April 2017.
Further reading
- Cao, Antonio; Galanello, Renzo (2010). "Beta-Thalassemia". In Pagon, Roberta A; Bird, Thomas D; Dolan, Cynthia R; Stephens, Karen; Adam, Margaret P. GeneReviews. PMID 20301599.
- Bahal, Raman; McNeer, Nicole Ali; Quijano, Elias; Liu, Yanfeng; Sulkowski, Parker; Turchick, Audrey; Lu, Yi-Chien; Bhunia, Dinesh C.; Manna, Arunava; Greiner, Dale L.; Brehm, Michael A.; Cheng, Christopher J.; López-Giráldez, Francesc; Ricciardi, Adele; Beloor, Jagadish; Krause, Diane S.; Kumar, Priti; Gallagher, Patrick G.; Braddock, Demetrios T.; Saltzman, W. Mark; Ly, Danith H.; Glazer, Peter M. (26 October 2016). "In vivo correction of anaemia in β-thalassemic mice by γPNA-mediated gene editing with nanoparticle delivery". Nature Communications. 7: 13304. Bibcode:2016NatCo...713304B. ISSN 2041-1723. PMC 5095181
 . PMID 27782131. doi:10.1038/ncomms13304. Retrieved 26 October 2016.
. PMID 27782131. doi:10.1038/ncomms13304. Retrieved 26 October 2016.