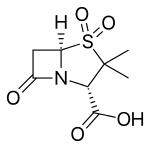
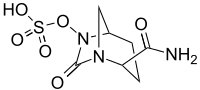
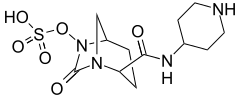
β-Lactamase inhibitor
Beta-lactamases are a family of enzymes involved in bacterial resistance to beta-lactam antibiotics. They act by breaking the beta-lactam ring that allows penicillin-like antibiotics to work. Strategies for combating this form of resistance have included the development of new beta-lactam antibiotics that are more resistant to cleavage and the development of the class of enzyme inhibitors called beta-lactamase inhibitors.[1] Although β-lactamase inhibitors have little antibiotic activity of their own,[2] they prevent bacterial degradation of beta-lactam antibiotics and thus extend the range of bacteria the drugs are effective against.
Medical uses
The most important use of beta-lactamase inhibitors is in the treatment of infections known or believed to be caused by gram-negative bacteria, as beta-lactamase production is an important contributor to beta-lactam resistance in these pathogens. In contrast, most beta-lactam resistance in gram-positive bacteria is due to variations in penicillin-binding proteins that lead to reduced binding to the beta-lactam.[3][4] The gram-positive pathogen Staphylococcus aureus produces beta-lactamases, but beta-lactamase inhibitors play a lesser role in treatment of these infections because the most resistant strains (methicillin-resistant Staphylococcus aureus) also use variant penicillin-binding proteins.[5][6]
Mechanism of action
The Ambler classification system groups known beta-lactamase enzymes into four groups according to sequence homology and presumed phylogenetic relationships. Classes A, C and D cleave beta-lactams by a multi-step mechanism analogous to the mechanism of serine proteases. Upon binding, a serine hydroxyl group in the beta-lactamase active site forms a transient covalent bond to the beta-lactam ring carbonyl group, cleaving the beta-lactam ring in the process. In a second step, nucleophilic attack by a water molecule cleaves the covalent bond between the enzyme and the carbonyl group of the erstwhile beta-lactam. This allows the degraded beta-lactam to diffuse away and frees up the enzyme to process additional beta-lactam molecules.
Currently available beta-lactamase inhibitors are effective against Ambler Class A beta-lactamases (tazobactam, clavulanate, and sulbactam) or against Ambler Class A, C and some Class D beta-lactamases (avibactam). Like beta-lactam antibiotics, they are processed by beta-lactamases to form an initial covalent intermediate. Unlike the case of beta-lactam antibiotics, the formed covalent intermediate is very stable. The persistence of the covalent bond between the beta-lactamase inhibitor and the beta-lactamase binding site deactivates the enzyme and prevents processing of beta-lactam antibiotics.[7]
Ambler Class B beta-lactams cleave beta-lactams by a mechanism similar to that of metalloproteases. As no covalent intermediate is formed, the mechanism of action of marketed beta-lactamase inhibitors is not applicable. Thus the spread of bacterial strains expressing metallo beta-lactamases such as the New Delhi metallo-beta-lactamase 1 has engendered considerable concern.[8]
Commonly used agents
Currently marketed β-lactamase inhibitors are not sold as individual drugs. Instead they are co-formulated with a β-lactam that has a similar serum half-life. This is done not only for dosing convenience, but also to minimize resistance development that might occur as a result of varying exposure to one or the other drug. The main classes of β-lactam antibiotics used to treat gram-negative bacterial infections include (in approximate order of intrinsic resistance to cleavage by β-lactamases) penicillins (especially aminopenicillins and ureidopenicillins), 3rd generation cephalosporins, and carbapenems. Individual β-lactamase variants may target one or many of these drug classes, and only a subset will be inhibited by a given β-lactamase inhibitor.[9] β-lactamase inhibitors expand the useful spectrum of these β-lactam antibiotics by inhibiting the β-lactamase enzymes produced by bacteria to deactivate them.[10]
- β-lactamase inhibitors with β-lactam core:
- Tebipenem is the first carbapenem to be administered orally in the form of Tebipenem-Pivoxil. Structural and kinetic studies of tebipenem is available with M.tuberculosis beta-lactamase (BlaC).[11]
- 6-Methylidene Penem2 is a newly designed beta-lactamase inhibitor and a very interesting one. After going inside the cell, when attacked by the enzyme beta-lactamase, it rearranges its molecular structure. In case of M. tuberculosis beta-lactamase, it exhibits 70 times higher activity than clavulanate. This rearrangement also makes it a good drug candidate to drug resistant beta-lactamases.[12]
- Boron based transition state inhibitors or BATSIs are very potent group of beta-lactamase inhibitors. A screen of a series of BATSIs against M. tuberculosis produces very interesting result. All the BATSIs with high inhibitory effects contain a benzoic carboxylic acid group. This is indeed a great break through in studying drug resistant beta-lactamases.[13]
- Clavulanic acid or clavulanate, usually combined with amoxicillin (Augmentin) or ticarcillin (Timentin)
- Sulbactam, usually combined with ampicillin (Unasyn) or Cefoperazone (Sulperazon)
- Tazobactam, usually combined with piperacillin (Zosyn) (Tazocin)
- Non-β-lactam β-lactamase inhibitors:
- Avibactam, approved in combination with ceftazidime (Avycaz), currently undergoing clinical trials for combination with ceftaroline
- Relebactam (previously known as MK-7655) is undergoing Phase III clinical trials as a treatment for pneumonia and bacterial infections (as of March 1, 2016).[14]
Beta-lactamase producing bacteria
Bacteria that can produce beta-lactamases include, but are not limited to:
- Mycobacterium tuberculosis
- MRSA(Methicillin-resistant Staphylococcus aureus)
- Staphylococcus
- Enterobacteriaceae
- Haemophilus influenzae
- Neisseria gonorrhoeae
- Klebsiella pneumoniae
- Citrobacter
- Morganella
Research
Some bacteria can produce extended spectrum β-lactamases (ESBLs) making the infection more difficult to treat and conferring additional resistance to penicillins, cephalosporins, and monobactams.[15] Boronic acid derivatives are currently under vast and extensive research as novel active site inhibitors for beta-lactamases because they contain a site that mimics the transition state that beta-lactams go through when undergoing hydrolysis via beta-lactamases. They have been found generally to fit well into the active site of many beta-lactamases and have the convenient property of being unable to be hydrolysed, and therefore rendered useless. This is a favorable drug design over many clinically used competing agents, because most of them, such as clavulonic acid, become hydrolysed, and are therefore only useful for a finite period of time. This generally causes the need for a higher concentration of competitive inhibitor than would be necessary in an unhydrolyzable inhibitor. Different boronic acid derivatives have to potential to be tailored to the many different isoforms of beta-lactamases, and therefore have the potential to reestablish potency of beta-lactam antibiotics.[16]
References
- ↑ Essack SY (2001). "The development of beta-lactam antibiotics in response to the evolution of beta-lactamases". Pharm. Res. 18 (10): 1391–9. PMID 11697463.
- ↑ "Beta-Lactamase Inhibitors". Department of Nursing of the Fort Hays State University College of Health and Life Sciences. October 2000. Archived from the original on 2007-09-27. Retrieved 2007-08-17.
- ↑ Georgopapadakou NH (1993). "Penicillin-binding proteins and bacterial resistance to beta-lactams". Antimicrob. Agents Chemother. 37 (10): 2045–53. PMC 192226
 . PMID 8257121. doi:10.1128/aac.37.10.2045.
. PMID 8257121. doi:10.1128/aac.37.10.2045. - ↑ Zapun A, Contreras-Martel C, Vernet T (2008). "Penicillin-binding proteins and beta-lactam resistance". FEMS Microbiol. Rev. 32 (2): 361–85. PMID 18248419. doi:10.1111/j.1574-6976.2007.00095.x.
- ↑ Curello J, MacDougall C (2014). "Beyond Susceptible and Resistant, Part II: Treatment of Infections Due to Gram-Negative Organisms Producing Extended-Spectrum β-Lactamases". J Pediatr Pharmacol Ther. 19 (3): 156–64. PMC 4187532
 . PMID 25309145. doi:10.5863/1551-6776-19.3.156.
. PMID 25309145. doi:10.5863/1551-6776-19.3.156. - ↑ Wolter DJ, Lister PD (2013). "Mechanisms of β-lactam resistance among Pseudomonas aeruginosa". Curr. Pharm. Des. 19 (2): 209–22. PMID 22894618. doi:10.2174/13816128130203.
- ↑ Drawz SM, Bonomo RA (2010). "Three decades of beta-lactamase inhibitors". Clin. Microbiol. Rev. 23 (1): 160–201. PMC 2806661
 . PMID 20065329. doi:10.1128/CMR.00037-09.
. PMID 20065329. doi:10.1128/CMR.00037-09. - ↑ Biedenbach D, Bouchillon S, Hackel M, Hoban D, Kazmierczak K, Hawser S, Badal R (2015). "Dissemination of NDM metallo-β-lactamase genes among clinical isolates of Enterobacteriaceae collected during the SMART global surveillance study from 2008 to 2012". Antimicrob. Agents Chemother. 59 (2): 826–30. PMC 4335866
 . PMID 25403666. doi:10.1128/AAC.03938-14.
. PMID 25403666. doi:10.1128/AAC.03938-14. - ↑ Drawz SM, Bonomo RA (2010). "Three decades of beta-lactamase inhibitors". Clin. Microbiol. Rev. 23 (1): 160–201. PMC 2806661
 . PMID 20065329. doi:10.1128/CMR.00037-09.
. PMID 20065329. doi:10.1128/CMR.00037-09. - ↑ Watson ID, Stewart MJ, Platt DJ (1988). "Clinical pharmacokinetics of enzyme inhibitors in antimicrobial chemotherapy". Clin Pharmacokinet. 15 (3): 133–64. PMID 3052984. doi:10.2165/00003088-198815030-00001.
- ↑ Hazra S, Xu H, Blanchard JS (Jun 2014). "Tebipenem, a new carbapenem antibiotic, is a slow substrate that inhibits the β-lactamase from Mycobacterium tuberculosis". Biochemistry. 53 (22): 3671–8. PMC 4053071
 . PMID 24846409. doi:10.1021/bi500339j.
. PMID 24846409. doi:10.1021/bi500339j. - ↑ Hazra S, Kurz SG, Wolff K, Nguyen L, Bonomo RA, Blanchard JS (Sep 2015). "Kinetic and Structural Characterization of the Interaction of 6-Methylidene Penem 2 with the β-Lactamase from Mycobacterium tuberculosis". Biochemistry. 54 (36): 5657–64. PMC 4795174
 . PMID 26237118. doi:10.1021/acs.biochem.5b00698.
. PMID 26237118. doi:10.1021/acs.biochem.5b00698. - ↑ Kurz SG, Hazra S, Bethel CR, Romagnoli C, Caselli E, Prati F, Blanchard JS, Bonomo RA (Mar 2015). "Inhibiting the β-Lactamase of Mycobacterium tuberculosis (Mtb) with Novel Boronic-Acid-Transition-State-Inhibitors (BATSIs)". ACS Infectious Diseases. 1: 234–42. doi:10.1021/acsinfecdis.5b00003.
- ↑ "Cilastatin/imipenem/relebactam — AdisInsight". Springer International Publishing AG. Retrieved 29 April 2016.
- ↑ Livermore, David M. (October 1995). "β-Lactamases in Laboratory and Clinical Resistance". Clinical Microbiology Reviews. 8 (4): 557–84. PMC 172876
 . PMID 8665470.
. PMID 8665470. - ↑ Leonard, David A.; Bonomo, Robert A.; Powers, Rachel (2012). "Class D β‑Lactamases: A Reappraisal after Five Decades". Accounts of Chemical Research. 46 (11): 2407–15. PMC 4018812
 . PMID 23902256. doi:10.1021/ar300327a.
. PMID 23902256. doi:10.1021/ar300327a.
External links
- Xu, Hua; Hazra, Saugata; Blanchard, John S. (2012). "NXL104 Irreversibly Inhibits the β-Lactamase from Mycobacterium tuberculosis". Biochemistry. 51 (22): 4551–7. PMC 3448018
 . PMID 22587688. doi:10.1021/bi300508r.
. PMID 22587688. doi:10.1021/bi300508r. - Kurz, Sebastian G.; Wolff, Kerstin A.; Hazra, Saugata; Bethel, Christopher R.; Hujer, Andrea M.; Smith, Kerri M.; Xu, Yan; Tremblay, Lee W.; Blanchard, John S.; Nguyen, Liem; Bonomo, Robert A. (2013). "Can Inhibitor-Resistant Substitutions in the Mycobacterium tuberculosis β-Lactamase BlaC Lead to Clavulanate Resistance?: a Biochemical Rationale for the Use of β-Lactam–β-Lactamase Inhibitor Combinations". Antimicrobial Agents and Chemotherapy. 57 (12): 6085–96. PMC 3837893
 . PMID 24060876. doi:10.1128/AAC.01253-13.
. PMID 24060876. doi:10.1128/AAC.01253-13.