Bergenin
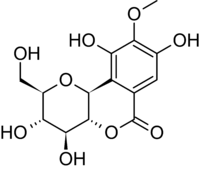 | |
| Names | |
|---|---|
| IUPAC name
(2R,3S,4S,4aR,10bS)-3,4,8,10-tetrahydroxy-2-(hydroxymethyl)-9-methoxy-3,
4,4a,10b-tetrahydro-2H-pyrano[3,2-c]isochromen-6-one | |
| Other names
Cuscutin | |
| Identifiers | |
| 3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.230.534 |
| PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C14H16O9 | |
| Molar mass | 328.27 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Bergenin, alias cuscutin, is trihydroxybenzoic acid glycoside. It is the C-glycoside of 4-O-methyl gallic acid. It possesses an O-demethylated derivative called norbergenin. These are chemical compounds and drugs of Ayurveda, commonly known as Paashaanbhed. It shows a potent immunomodulatory effect.[1]
Bergenin can be isolated from Bergenia species like Bergenia ciliata and Bergenia ligulata,[2] from rhizomes of Bergenia stracheyi. It is also found in the stem bark of Dryobalanops aromatica,[3] in Ardisia elliptica and in Mallotus japonicus.[4]
References
- ↑ Nazir, N.; Koul, S.; Qurishi, M. A.; Taneja, S. C.; Ahmad, S. F.; Bani, S.; Qazi, G. N. (2007). "Immunomodulatory effect of bergenin and norbergenin against adjuvant-induced arthritis—A flow cytometric study". Journal of Ethnopharmacology. 112 (2): 401–405. PMID 17408893. doi:10.1016/j.jep.2007.02.023.
- ↑ Dhalwal, K.; Shinde, V. M.; Biradar, Y. S.; Mahadik, K. R. (2008). "Simultaneous quantification of bergenin, catechin, and gallic acid from Bergenia ciliata and Bergenia ligulata by using thin-layer chromatography". Journal of Food Composition and Analysis. 21 (6): 496–500. doi:10.1016/j.jfca.2008.02.008. INIST:20528090.
- ↑ Wibowo, A.; Ahmat, N.; Hamzah, A. S.; Sufian, A. S.; Ismail, N. H.; Ahmad, R.; Jaafar, F. M.; Takayama, H. (2011). "Malaysianol A, a new trimer resveratrol oligomer from the stem bark of Dryobalanops aromatica". Fitoterapia. 82 (4): 676–681. PMID 21338657. doi:10.1016/j.fitote.2011.02.006.
- ↑ Hepatoprotective effects of bergenin, a major constituent of Mallotus japonicus, on carbon tetrachloride-intoxicated rats. Lim HwaKyung, Kim HackSeang, Choi HongSerck, Oh SeiKwan and Choi JongWon, Journal of Ethnopharmacology, 2000, Volume 72 , Number 3, pages 469-474, doi:10.1016/S0378-8741(00)00260-9
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.