Behavioural genetics
Behavioural genetics (Commonwealth English) or behavioral genetics (American English), also referred to as behaviour genetics, is a field of scientific research that uses genetic methods to investigate the nature and origins of individual differences in behaviour. While the name "behavioural genetics" connotes a focus on genetic influences, the field broadly investigates genetic and environmental influences, using research designs that allow removal of the confounding of genes and environment. Behavioural genetics was founded as a scientific discipline by Francis Galton in the late 19th century, only to be discredited through association with eugenics movements before and during World War II. In the latter half of the 20th century, the field saw renewed prominence with research on inheritance of behaviour and mental illness in humans (typically using twin and family studies), as well as research on genetically informative model organisms through selective breeding and crosses. In the late 20th and early 21st centuries, technological advances in molecular genetics made it possible to measure and modify the genome directly. This led to major advances in model organism research (e.g., knockout mice) and in human studies (e.g., genome-wide association studies), leading to new scientific discoveries.
Findings from behavioural genetic research have broadly impacted modern understanding of the role of genetic and environmental influences on behaviour. These include evidence that nearly all researched behaviors are under a significant degree of genetic influence, and that influence tends to increase as individuals develop into adulthood. Further, most researched human behaviours are influenced by a very large number of genes and the individual effects of these genes are very small. Environmental influences also play a strong role, but they tend to make family members more different from one another, not more similar.
History

Selective breeding and the domestication of animals is perhaps the earliest evidence that humans considered the idea that individual differences in behaviour could be due to natural causes.[1] Plato and Aristotle each speculated on the basis and mechanisms of inheritance of behavioural characteristics.[2] Plato, for example, argued in The Republic that selective breeding among the citizenry to encourage the development of some traits and discourage others, what today might be called eugenics, was to be encouraged in the pursuit of an ideal society.[2][3] Behavioural genetic concepts also existed during the English renaissance, where William Shakespeare perhaps first coined the terms "nature" versus "nurture" in The Tempest, where he wrote in Act IV, Scene I, that Caliban was "A devil, a born devil, on whose nature Nurture can never stick".[3][4]
Modern-day behavioural genetics began with Sir Francis Galton, a nineteenth-century intellectual and cousin of Charles Darwin.[3] Galton was a polymath who studied many things, including the heritability of human abilities and mental characteristics. One of Galton's investigations involved a large pedigree study of social and intellectual achievement in the English upper class. In 1869, 10 years after Darwin's Origin of the species, Galton published his results in Hereditary Genius.[5] In this work, Galton found that the rate of "eminence" was highest among close relatives of eminent individuals, and decreased as the degree of relationship to eminent individuals decreased. While Galton could not rule out the role of environmental influences on eminence, a fact which he acknowledged, the study served to initiate an important debate about the relative roles of genes and environment on behavioural characteristics. Through his work, Galton also "introduced multivariate analysis and paved the way towards modern Bayesian statistics" that are used throughout the sciences—launching what has been dubbed the "Statistical Enlightenment".[6]

The field of behavioural genetics, as founded by Galton, was ultimately undermined by another of Galton's intellectual contributions, the founding of the eugenics movement in 20th century society.[3] The primary idea behind eugenics was to use selective breeding combined with knowledge about the inheritance of behaviour to improve the human species.[3] The eugenics movement was subsequently discredited by scientific corruption and genocidal actions in Nazi Germany. Behavioural genetics was thereby discredited through its association to eugenics.[3] The field once again gained status as a distinct scientific discipline through the publication of early texts on behavioural genetics, such as Calvin S. Hall's 1951 book chapter on behavioural genetics, in which he introduced the term "psychogenetics",[7] which enjoyed some limited popularity in the 1960s and 1970s.[8][9] However, it eventually disappeared from usage in favour of "behaviour genetics".
The start of behavior genetics as a well-identified field was marked by the publication in 1960 of the book Behavior Genetics by John L. Fuller and William Robert (Bob) Thompson.[1][10] It is widely accepted now that many if not most behaviours in animals and humans are under significant genetic influence, although the extent of genetic influence for any particular trait can differ widely.[11][12] A decade later, in February 1970, the first issue of the journal Behavior Genetics was published and in 1972 the Behavior Genetics Association was formed with Theodosius Dobzhansky elected as the association's first president. The field has since grown and diversified, touching many scientific disciplines.[3][13]
Behavioural genetic research and findings have at times been controversial. Some of this controversy has arisen because behavioural genetic findings can challenge societal beliefs about the nature of human behaviour and abilities, other controversies have arisen due to misunderstandings of behavioural genetic research, whether by the lay public or the researchers themselves.[3] Major areas of controversy have included genetic research on topics such as racial differences, intelligence, violence, and human sexuality.[14]
Methods
The primary goal of behavioural genetics is to investigate the nature and origins of individual differences in behaviour.[3] A wide variety of different methodological approaches are used in behavioral genetic research,[15] only a few of which are outlined below.
Animal studies
In animal research selection experiments have often been employed. For example, laboratory house mice have been bred for open-field behaviour,[16] thermoregulatory nesting,[17] and voluntary wheel-running behaviour.[18] A range of methods in these designs are covered on those pages.
Behavioural geneticists using model organisms employ a range of molecular techniques to alter, insert, or delete genes. These techniques include knockouts, floxing, gene knockdown, or genome editing using methods like CRISPR-Cas9.[19] These techniques allow behavioural geneticists different levels of control in the model organism's genome, to evaluate the molecular, physiological, or behavioural outcome of genetic changes.[20]
Twin and family studies

One research design used in behavioural genetic research are variations on family designs (also known as pedigree designs), including twin studies and adoption studies.[15] Quantitative genetic modelling of individuals with known genetic relationships (e.g., parent-child, sibling, dizygotic and monozygotic twins) allows one to estimate to what extent genes and environment contribute to phenotypic differences among individuals.[21] The basic intuition of the twin study is that monozygotic twins share 100% of their genome and dizygotic twins share, on average, 50% of their segregating genome. Thus, differences between the two members of a monozygotic twin pair can only be due to differences in their environment, whereas dizygotic twins will differ from one another due to environment as well as genes. Under this simplistic model, if dizygotic twins differ more than monozygotic twins it can only be attributable to genetic influences. An important assumption of the twin model is the equal environment assumption[22] that monozygotic twins have the same shared environmental experiences as dizygotic twins. If, for example, monozygotic twins tend to have more similar experiences than dizygotic twins—and these experiences themselves are not genetically mediated through gene-environment correlation mechanisms—then monozygotic twins will tend to be more similar to one another than dizygotic twins for reasons that have nothing to do with genes.[23]
Twin studies of monozygotic and dizygotic twins use a biometrical formulation to describe the influences on twin similarity and to infer heritability.[21][24] The formulation rests on the basic observation that the variance in a phenotype is due to two sources, genes and environment. More formally, , where is the phenotype, is the effect of genes, is the effect of the environment, and is a gene by environment interaction. The term can be expanded to include additive (), dominance (), and epistatic () genetic effects. Similarly, the environmental term can be expanded to include shared environment () and non-shared environment (), which includes any measurement error. Dropping the gene by environment interaction for simplicity (typical in twin studies) and fully decomposing the and terms, we now have . Twin research then models the similarity in monozygotic twins and dizogotic twins using simplified forms of this decomposition, shown in the table.[21]
| Type of relationship | Full decomposition | Falconer's decomposition |
|---|---|---|
| Perfect similarity between siblings | ||
| Monozygotic twin correlation() | ||
| Dizygotic twin correlation () | ||
| Where is an unknown (probably very small) quantity. | ||
The simplified Falconer formulation can then be used to derive estimates of , , and . Rearranging and substituting the and equations one can obtain an estimate of the additive genetic variance, or heritability, , the non-shared environmental effect and, finally, the shared environmental effect .[21] The Falconer formulation is presented here to illustrate how the twin model works. Modern approaches use maximum likelihood to estimate the genetic and environmental variance components.[25]
Measured genetic variants
The Human Genome Project has allowed scientists to directly genotype the sequence of human DNA nucleotides.[26] Once genotyped, genetic variants can be tested for association with a behavioural phenotype, such as mental disorder, cognitive ability, personality, and so on.[27]
- Candidate Genes. One popular approach has been to test for association candidate genes with behavioural phenotypes, where the candidate gene is selected based on some a priori theory about biological mechanisms involved in the manifestation of a behavioural trait or phenotype.[28] In general, such studies have proven difficult to broadly replicate[29][30] and there has been concern raised that the false positive rate in this type of research is high.[28][31]
- Genome-wide association studies. In genome-wide association studies, researchers test the relationship of millions of genetic polymorphisms with behavioural phenotypes across the genome.[27] This approach to genetic association studies is largely atheoretical, and typically not guided by a particular biological hypothesis regarding the phenotype.[27] Genetic association findings for behavioural traits and psychiatric disorders have been found to be highly polygenic (involving many small genetic effects).[32][33][34][35][36]
- SNP heritability and co-heritability. Recently, researchers have begun to use similarity between classically unrelated people at their measured single nucleotide polymorphisms (SNPs) to estimate genetic variation or covariation that is tagged by SNPs, using mixed effects models implemented in software such as Genome-wide complex trait analysis (GCTA).[37][38] To do this, researchers find the average genetic relatedness over all SNPs between all individuals in a (typically large) sample, and use Haseman-Elston regression or restricted maximum likelihood to estimate the genetic variation that is "tagged" by, or predicted by, the SNPs. The proportion of phenotypic variation that is accounted for by the genetic relatedness has been called "SNP heritability".[39] Intuitively, SNP heritability increases to the degree that phenotypic similarity is predicted by genetic similarity at measured SNPs, and is expected to be lower than the true narrow-sense heritability to the degree that measured SNPs fail to tag (typically rare) causal variants.[40] The value of this method is that it is an independent way to estimate heritability that does not require the same assumptions as those in twin and family studies, and that it gives insight into the allelic frequency spectrum of the causal variants underlying trait variation.[41]
Quasi-experimental designs
Some behavioural genetic designs are useful not to understand genetic influences on behaviour, but to control for genetic influences to test environmentally-mediated influences on behaviour.[42] Such behavioural genetic designs may be considered a subset of natural experiments,[43] quasi-experiments that attempt to take advantage of naturally occurring situations that mimic true experiments by providing some control over an independent variable. Natural experiments can be particularly useful when experiments are infeasible, due to practical or ethical limitations.[43]
A general limitation of observational studies is that the relative influences of genes and environment are confounded. A simple demonstration of this fact is that measures of 'environmental' influence are heritable.[44] Thus, observing a correlation between an environmental risk factor and a health outcome is not necessarily evidence for environmental influence on the health outcome. Similarly, in observational studies of parent-child behavioural transmission, for example, it is impossible to know if the transmission is due to genetic or environmental influences, due to the problem of passive gene-environment correlation.[43] The simple observation that the children of parents who use drugs are more likely to use drugs as adults does not indicate why the children are more likely to use drugs when they grow up. It could be because the children are modelling their parents' behaviour. Equally plausible, it could be that the children inherited drug-use-predisposing genes from their parent, which put them at increased risk for drug use as adults regardless of their parents' behaviour. Adoption studies, which parse the relative effects of rearing environment and genetic inheritance, find a small to negligible effect of rearing environment on smoking, alcohol, and marijuana use in adopted children,[45] but a larger effect of rearing environment on harder drug use.[46]
Other behavioural genetic designs include discordant twin studies,[42] children of twins designs,[47] and Mendelian randomization.[48]
General findings
There are many broad conclusions to be drawn from behavioural genetic research about the nature and origins of behaviour.[3][49] Three major conclusions include: 1) all behavioural traits and disorders are influenced by genes; 2) environmental influences tend to make members of the same family more different, rather than more similar; and 3) the influence of genes tends to increase in relative importance as individuals age.[3]
Genetic influences on behaviour are pervasive
It is clear from multiple lines of evidence that all researched behavioural traits and disorders are influenced by genes; that is, they are heritable. The single largest source of evidence comes from twin studies, where it is routinely observed that monozygotic (identical) twins are more similar to one another than are same-sex dizygotic (fraternal) twins.[11][12]
The conclusion that genetic influences are pervasive has also been observed in research designs that do not depend on the assumptions of the twin method. Adoption studies show that adoptees are routinely more similar to their biological relatives than their adoptive relatives for a wide variety of traits and disorders.[3] In the Minnesota Study of Twins Reared Apart, monozygotic twins separated shortly after birth were reunited in adulthood.[50] These adopted, reared-apart twins were as similar to one another as were twins reared together on a wide range of measures including general cognitive ability, personality, religious attitudes, and vocational interests, among others.[50] Approaches using genome-wide genotyping have allowed researchers to measure genetic relatedness between individuals and estimate heritability based on millions of genetic variants. Methods exist to test whether the extent of genetic similarity (aka, relatedness) between nominally unrelated individuals (individuals who are not close or even distant relatives) is associated with phenotypic similarity.[38] Such methods do not rely on the same assumptions as twin or adoption studies, and routinely find evidence for heritability of behavioural traits and disorders.[34][36][51]
Nature of environmental influence
Just as all researched human behavioural phenotypes are influenced by genes (i.e., are heritable), all such phenotypes are also influenced by the environment.[11][49] The basic fact that monozygotic twins are genetically identical but are never perfectly concordant for psychiatric disorder or perfectly correlated for behavioural traits, indicates that the environment shapes human behaviour.[49]
The nature of this environmental influence, however, is such that it tends to make individuals in the same family more different from one another, not more similar to one another.[3] That is, estimates of shared environmental effects () in human studies are small, negligible, or zero for the vast majority of behavioural traits and psychiatric disorders, whereas estimates of non-shared environmental effects () are moderate to large.[11] From twin studies is typically estimated at 0 because the correlation () between monozygotic twins is at least twice the correlation () for dizygotic twins. When using the Falconer variance decomposition () this difference between monozygotic and dizygotic twin similarity results in an estimated . It is important to note that the Falconer decomposition is simplistic.[21] It removes the possible influence of dominance and epistatic effects which, if present, will tend to make monozygotic twins more similar than dizygotic twins and mask the influence of shared environmental effects.[21] This is a limitation of the twin design for estimating . However, the general conclusion that shared environmental effects are negligible does not rest on twin studies alone. Adoption research also fails to find large () components; that is, adoptive parents and their adopted children tend to show much less resemblance to one another than the adopted child and his or her non-rearing biological parent.[3] In studies of adoptive families with at least one biological child and one adopted child, the sibling resemblance also tends be nearly zero for most traits that have been studied.[11][52]
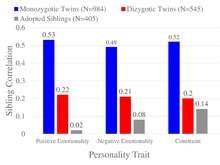
The figure provides an example from personality research, where twin and adoption studies converge on the conclusion of zero to small influences of shared environment on broad personality traits measured by the Multidimensional Personality Questionnaire including positive emotionality, negative emotionality, and constraint.[53]
Given the conclusion that all researched behavioural traits and psychiatric disorders are heritable, biological siblings will always tend to be more similar to one another than will adopted siblings. However, for some traits, especially when measured during adolescence, adopted siblings do show some significant similarity (e.g., correlations of .20) to one another. Traits that have been demonstrated to have significant shared environmental influences include internalizing and externalizing psychopathology,[54] substance use[55] and dependence,[46] and intelligence.[55]
Nature of genetic influence
Genetic effects on human behavioural outcomes can be described in multiple ways.[21] One way to describe the effect is in terms of how much variance in the behaviour can be accounted for by alleles in the genetic variant, otherwise known as the coefficient of determination or . An intuitive way to think about is that it describes the extent to which the genetic variant makes individuals, who harbour different alleles, different from one another on the behavioural outcome. A complementary way to describe effects of individual genetic variants is in how much change one expects on the behavioural outcome given a change in the number of risk alleles an individual harbours, often denoted by the Greek letter (denoting the slope in a regression equation), or, in the case of binary disease outcomes by the odds ratio of disease given allele status. Note the difference: describes the population-level effect of alleles within a genetic variant; or describe the effect of having a risk allele on the individual who harbours it, relative to an individual who does not harbour a risk allele.[56]
When described on the metric, the effects of individual genetic variants on complex human behavioural traits and disorders are vanishingly small, with each variant accounting for of variation in the phenotype.[3] This fact has been discovered primarily through genome-wide association studies of complex behavioural phenotypes, including results on substance use,[57][58] personality,[59] fertility,[60] schizophrenia,[33] depression,[59][61] and endophenotypes including brain structure[62] and function.[63] There are a small handful of replicated and robustly studied exceptions to this rule, including the effect of APOE on Alzheimer's disease,[64] and CHRNA5 on smoking behaviour,[57] and ALDH2 (in individuals of East Asian ancestry) on alcohol use.[65]
On the other hand, when assessing effects according to the metric, there are a large number of genetic variants that have very large effects on complex behavioural phenotypes. The risk alleles within such variants are exceedingly rare, such that their large behavioural effects impact only a small number of individuals. Thus, when assessed at a population level using the metric, they account for only a small amount of the differences in risk between individuals in the population. Examples include variants within APP that result in familial forms of severe early onset Alzheimer's disease but affect only relatively few individuals. Compare this to risk alleles within APOE, which pose much smaller risk compared to APP, but are far more common and therefore affect a much greater proportion of the population.[66]
Finally, there are classical behavioural disorders that are genetically simple in their etiology, such as Huntington's disease. Huntington's is caused by a single autosomal dominant variant in the HTT gene, which is the only variant that accounts for any differences among individuals in their risk for developing the disease, assuming they live long enough.[67] In the case of genetically simple and rare diseases such as Huntington's, the variant and the are simultaneously large.[56]
See also
- Adoption study
- Behavior Genetics
- Behavior Genetics Association
- Behavioural neurogenetics
- Biocultural evolution
- Evolutionary psychology
- Genes, Brain and Behavior
- Genome-wide association study
- Human behaviour genetics
- International Behavioural and Neural Genetics Society
- International Society of Psychiatric Genetics
- Journal of Neurogenetics
- Molecular genetics
- Nature versus nurture
- Psychiatric genetics
- Psychiatric Genetics
- Quantitative genetics
- Twin study
References
- 1 2 Loehlin JC (2009). "History of behavior genetics". In Kim Y. Handbook of behavior genetics (1 ed.). New York, NY: Springer. ISBN 978-0-387-76726-0. doi:10.1007/978-0-387-76727-7_1.
- 1 2 Maxson SC (30 August 2006). "A History of Behavior Genetics". In Jones BC, Mormede P. Neurobehavioral Genetics: Methods and Applications, Second Edition. CRC Press. ISBN 978-1-4200-0356-7.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 McGue M, Gottesman II (2015). "Behavior Genetics". The Encyclopedia of Clinical Psychology. pp. 1–11. doi:10.1002/9781118625392.wbecp578.
- ↑ Vaughan VM, Vaughan AT (1999). The Tempest. The Arden Shakespeare (Third ed.). The Arden Shakespeare. p. 60. ISBN 978-1-903436-08-0.
- ↑ Hereditary Genius: An Inquiry into Its Laws and Consequences. London: MacMillan and Co. 1869.
- ↑ Stigler SM (July 2010). "Darwin, Galton and the Statistical Enlightenment". Journal of the Royal Statistical Society, Series A. 173 (3): 469–482. doi:10.1111/j.1467-985X.2010.00643.x.
- ↑ Hall CS (1951). "The genetics of behavior". In Stevens SS. Handbook of Experimental Psychology. New York: John Wiley and Sons. pp. 304–329.
- ↑ Grigorenko EL, Ravich-Shcherbo I (1997). "Russian psychogenetics". In Grigorenko EL. Psychology of Russia: Past, Present, Future. Commack, NY: Nova Science. pp. 83–124.
- ↑ Broadhurst PL (July 1969). "Psychogenetics of emotionality in the rat". Annals of the New York Academy of Sciences. 159 (3): 806–24. Bibcode:1969NYASA.159..806B. PMID 5260300. doi:10.1111/j.1749-6632.1969.tb12980.x.
- ↑ Fuller JL, Thompson WR (1960). Behavior Genetics. New York: John Wiley and Sons.
- 1 2 3 4 5 Polderman TJ, Benyamin B, de Leeuw CA, Sullivan PF, van Bochoven A, Visscher PM, Posthuma D (July 2015). "Meta-analysis of the heritability of human traits based on fifty years of twin studies". Nature Genetics. 47 (7): 702–9. PMID 25985137. doi:10.1038/ng.3285.
- 1 2 Turkheimer E (2000). "Three Laws of Behavior Genetics and What They Mean" (PDF). Current Directions in Psychological Science. 9: 160–164. doi:10.1111/1467-8721.00084.
- ↑ Ayorech Z, Selzam S, Smith-Woolley E, Knopik VS, Neiderhiser JM, DeFries JC, Plomin R (September 2016). "Publication Trends Over 55 Years of Behavioral Genetic Research". Behavior Genetics. 46 (5): 603–7. PMID 26992731. doi:10.1007/s10519-016-9786-2.
- ↑ Check Hayden, Erika (2013). "Ethics: Taboo genetics". Nature. 502 (7469): 26–28. ISSN 0028-0836. PMID 24091964. doi:10.1038/502026a.
- 1 2 Plomin R, DeFries JC, Knopik VS, Neiderhiser JM (24 September 2012). Behavioral Genetics. Worth Publishers. ISBN 978-1-4292-4215-8. Lay summary (4 September 2013).
- ↑ DeFries JC, Hegmann JP, Halcomb RA (August 1974). "Response to 20 generations of selection for open-field activity in mice". Behavioral Biology. 11 (4): 481–95. PMID 4415597. doi:10.1016/s0091-6773(74)90800-1.
- ↑ Lynch CB (November 1980). "Response to divergent selection for nesting behavior in Mus musculus". Genetics. 96 (3): 757–65. PMC 1214374
 . PMID 7196362.
. PMID 7196362. - ↑ Swallow JG, Carter PA, Garland T (May 1998). "Artificial selection for increased wheel-running behavior in house mice". Behavior Genetics. 28 (3): 227–37. PMID 9670598. doi:10.1023/A:1021479331779.
- ↑ Heidenreich M, Zhang F (January 2016). "Applications of CRISPR-Cas systems in neuroscience". Nature Reviews. Neuroscience. 17 (1): 36–44. PMC 4899966
 . PMID 26656253. doi:10.1038/nrn.2015.2.
. PMID 26656253. doi:10.1038/nrn.2015.2. - ↑ Singh P, Schimenti JC, Bolcun-Filas E (January 2015). "A mouse geneticist's practical guide to CRISPR applications". Genetics. 199 (1): 1–15. PMC 4286675
 . PMID 25271304. doi:10.1534/genetics.114.169771.
. PMID 25271304. doi:10.1534/genetics.114.169771. - 1 2 3 4 5 6 7 8 Douglas Scott Falconer (1989). Introduction to quantitative genetics. Longman, Scientific & Technical. ISBN 978-0-470-21162-5.
- ↑ Eaves L, Foley D, Silberg J (2003). "Has the "Equal Environments" assumption been tested in twin studies?". Twin Research : the Official Journal of the International Society for Twin Studies. 6 (6): 486–9. PMID 14965458. doi:10.1375/136905203322686473.
- ↑ Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ (January 1993). "A test of the equal-environment assumption in twin studies of psychiatric illness". Behavior Genetics. 23 (1): 21–7. PMID 8476388. doi:10.1007/BF01067551.
- ↑ Jinks JL, Fulker DW (1970). "Comparison of the biometrical genetical, MAVA, and classical approaches to the analysis of the human behavior.". Psychological Bulletin. 73: 311–349. PMID 5528333. doi:10.1037/h0029135.
- ↑ Martin NG, Eaves LJ (February 1977). "The genetical analysis of covariance structure". Heredity. 38 (1): 79–95. PMID 268313. doi:10.1038/hdy.1977.9.
- ↑ Lander ES (February 2011). "Initial impact of the sequencing of the human genome". Nature. 470 (7333): 187–97. PMID 21307931. doi:10.1038/nature09792.
- 1 2 3 McCarthy MI, Abecasis GR, Cardon LR, Goldstein DB, Little J, Ioannidis JP, Hirschhorn JN (May 2008). "Genome-wide association studies for complex traits: consensus, uncertainty and challenges". Nature Reviews Genetics. 9 (5): 356–69. PMID 18398418. doi:10.1038/nrg2344.
- 1 2 Duncan LE, Keller MC (October 2011). "A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry". The American Journal of Psychiatry. 168 (10): 1041–9. PMC 3222234
 . PMID 21890791. doi:10.1176/appi.ajp.2011.11020191.
. PMID 21890791. doi:10.1176/appi.ajp.2011.11020191. - ↑ Farrell MS, Werge T, Sklar P, Owen MJ, Ophoff RA, O'Donovan MC, Corvin A, Cichon S, Sullivan PF (May 2015). "Evaluating historical candidate genes for schizophrenia". Molecular Psychiatry. 20 (5): 555–62. PMC 4414705
 . PMID 25754081. doi:10.1038/mp.2015.16.
. PMID 25754081. doi:10.1038/mp.2015.16. - ↑ Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG (November 2001). "Replication validity of genetic association studies". Nature Genetics. 29 (3): 306–9. PMID 11600885. doi:10.1038/ng749.
- ↑ Colhoun HM, McKeigue PM, Davey Smith G (March 2003). "Problems of reporting genetic associations with complex outcomes". Lancet. 361 (9360): 865–72. PMID 12642066. doi:10.1016/S0140-6736(03)12715-8.
- ↑ Visscher PM, Brown MA, McCarthy MI, Yang J (January 2012). "Five years of GWAS discovery". American Journal of Human Genetics. 90 (1): 7–24. PMC 3257326
 . PMID 22243964. doi:10.1016/j.ajhg.2011.11.029.
. PMID 22243964. doi:10.1016/j.ajhg.2011.11.029. - 1 2 "Biological insights from 108 schizophrenia-associated genetic loci". Nature. 511 (7510): 421–7. July 2014. PMC 4112379
 . PMID 25056061. doi:10.1038/nature13595.
. PMID 25056061. doi:10.1038/nature13595. - 1 2 Lee SH, DeCandia TR, Ripke S, Yang J, Sullivan PF, Goddard ME, Keller MC, Visscher PM, Wray NR (February 2012). "Estimating the proportion of variation in susceptibility to schizophrenia captured by common SNPs". Nature Genetics. 44 (3): 247–50. PMC 3327879
 . PMID 22344220. doi:10.1038/ng.1108.
. PMID 22344220. doi:10.1038/ng.1108. - ↑ Sullivan PF, Daly MJ, O'Donovan M (July 2012). "Genetic architectures of psychiatric disorders: the emerging picture and its implications". Nature Reviews Genetics. 13 (8): 537–51. PMC 4110909
 . PMID 22777127. doi:10.1038/nrg3240.
. PMID 22777127. doi:10.1038/nrg3240. - 1 2 de Moor MH, van den Berg SM, Verweij KJ, Krueger RF, Luciano M, Arias Vasquez A, et al. (July 2015). "Meta-analysis of Genome-wide Association Studies for Neuroticism, and the Polygenic Association With Major Depressive Disorder". JAMA Psychiatry. 72 (7): 642–50. PMC 4667957
 . PMID 25993607. doi:10.1001/jamapsychiatry.2015.0554.
. PMID 25993607. doi:10.1001/jamapsychiatry.2015.0554. - ↑ Yang J, Benyamin B, McEvoy BP, Gordon S, Henders AK, Nyholt DR, Madden PA, Heath AC, Martin NG, Montgomery GW, Goddard ME, Visscher PM (July 2010). "Common SNPs explain a large proportion of the heritability for human height". Nature Genetics. 42 (7): 565–9. PMC 3232052
 . PMID 20562875. doi:10.1038/ng.608.
. PMID 20562875. doi:10.1038/ng.608. - 1 2 Yang J, Lee SH, Goddard ME, Visscher PM (January 2011). "GCTA: a tool for genome-wide complex trait analysis". American Journal of Human Genetics. 88 (1): 76–82. PMC 3014363
 . PMID 21167468. doi:10.1016/j.ajhg.2010.11.011.
. PMID 21167468. doi:10.1016/j.ajhg.2010.11.011. - ↑ Lee SH, Yang J, Chen GB, Ripke S, Stahl EA, Hultman CM, Sklar P, Visscher PM, Sullivan PF, Goddard ME, Wray NR (2013). "Estimation of SNP heritability from dense genotype data". American Journal of Human Genetics. 93 (6): 1151–5. PMC 3852919
 . PMID 24314550. doi:10.1016/j.ajhg.2013.10.015.
. PMID 24314550. doi:10.1016/j.ajhg.2013.10.015. - ↑ Visscher PM, Yang J, Goddard ME (2010). "A commentary on 'common SNPs explain a large proportion of the heritability for human height' by Yang et al. (2010)". Twin Research and Human Genetics : the Official Journal of the International Society for Twin Studies. 13 (6): 517–24. PMID 21142928. doi:10.1375/twin.13.6.517.
- ↑ Wray NR, Lee SH, Mehta D, Vinkhuyzen AA, Dudbridge F, Middeldorp CM (2014). "Research review: Polygenic methods and their application to psychiatric traits". Journal of Child Psychology and Psychiatry, and Allied Disciplines. 55 (10): 1068–87. PMID 25132410. doi:10.1111/jcpp.12295.
- 1 2 McGue M, Osler M, Christensen K (September 2010). "Causal Inference and Observational Research: The Utility of Twins". Perspectives on Psychological Science. 5 (5): 546–56. PMC 3094752
 . PMID 21593989. doi:10.1177/1745691610383511.
. PMID 21593989. doi:10.1177/1745691610383511. - 1 2 3 Rutter M (December 2007). "Proceeding From Observed Correlation to Causal Inference: The Use of Natural Experiments". Perspectives on Psychological Science. 2 (4): 377–95. PMID 26151974. doi:10.1111/j.1745-6916.2007.00050.x.
- ↑ Kendler KS, Baker JH (May 2007). "Genetic influences on measures of the environment: a systematic review". Psychological Medicine. 37 (5): 615–26. PMID 17176502. doi:10.1017/S0033291706009524.
- ↑ Keyes M, Legrand LN, Iacono WG, McGue M (October 2008). "Parental smoking and adolescent problem behavior: an adoption study of general and specific effects". The American Journal of Psychiatry. 165 (10): 1338–44. PMC 2597022
 . PMID 18676589. doi:10.1176/appi.ajp.2008.08010125.
. PMID 18676589. doi:10.1176/appi.ajp.2008.08010125. - 1 2 Kendler KS, Sundquist K, Ohlsson H, Palmér K, Maes H, Winkleby MA, Sundquist J (July 2012). "Genetic and familial environmental influences on the risk for drug abuse: a national Swedish adoption study". Archives of General Psychiatry. 69 (7): 690–7. PMC 3556483
 . PMID 22393206. doi:10.1001/archgenpsychiatry.2011.2112.
. PMID 22393206. doi:10.1001/archgenpsychiatry.2011.2112. - ↑ D'Onofrio BM, Turkheimer EN, Eaves LJ, Corey LA, Berg K, Solaas MH, Emery RE (November 2003). "The role of the children of twins design in elucidating causal relations between parent characteristics and child outcomes". Journal of Child Psychology and Psychiatry, and Allied Disciplines. 44 (8): 1130–44. PMID 14626455. doi:10.1111/1469-7610.00196.
- ↑ Smith GD, Ebrahim S (February 2004). "Mendelian randomization: prospects, potentials, and limitations". International Journal of Epidemiology. 33 (1): 30–42. PMID 15075143. doi:10.1093/ije/dyh132.
- 1 2 3 Plomin R, DeFries JC, Knopik VS, Neiderhiser JM (January 2016). "Top 10 Replicated Findings From Behavioral Genetics". Perspectives on Psychological Science. 11 (1): 3–23. PMID 26817721. doi:10.1177/1745691615617439.
- 1 2 Bouchard TJ, Lykken DT, McGue M, Segal NL, Tellegen A (October 1990). "Sources of human psychological differences: the Minnesota Study of Twins Reared Apart". Science. 250 (4978): 223–8. PMID 2218526. doi:10.1126/science.2218526.
- ↑ Plomin R, Haworth CM, Meaburn EL, Price TS, Davis OS (April 2013). "Common DNA markers can account for more than half of the genetic influence on cognitive abilities". Psychological Science. 24 (4): 562–8. PMC 3652710
 . PMID 23501967. doi:10.1177/0956797612457952.
. PMID 23501967. doi:10.1177/0956797612457952. - ↑ Plomin R, Daniels D (June 2011). "Why are children in the same family so different from one another?". International Journal of Epidemiology. 40 (3): 563–82. PMC 3147063
 . PMID 21807642. doi:10.1093/ije/dyq148.
. PMID 21807642. doi:10.1093/ije/dyq148. - ↑ Matteson LK, McGue M, Iacono WG (November 2013). "Shared environmental influences on personality: a combined twin and adoption approach". Behavior Genetics. 43 (6): 491–504. PMC 3868213
 . PMID 24065564. doi:10.1007/s10519-013-9616-8.
. PMID 24065564. doi:10.1007/s10519-013-9616-8. - ↑ Burt SA (July 2009). "Rethinking environmental contributions to child and adolescent psychopathology: a meta-analysis of shared environmental influences". Psychological Bulletin. 135 (4): 608–37. PMID 19586164. doi:10.1037/a0015702.
- 1 2 Buchanan JP, McGue M, Keyes M, Iacono WG (September 2009). "Are there shared environmental influences on adolescent behavior? Evidence from a study of adoptive siblings". Behavior Genetics. 39 (5): 532–40. PMC 2858574
 . PMID 19626434. doi:10.1007/s10519-009-9283-y.
. PMID 19626434. doi:10.1007/s10519-009-9283-y. - 1 2 Bland JM (2000). "Statistics Notes: The odds ratio". BMJ. 320 (7247): 1468–1468. ISSN 0959-8138. doi:10.1136/bmj.320.7247.1468.
- 1 2 Thorgeirsson TE, Gudbjartsson DF, Surakka I, Vink JM, Amin N, Geller F, et al. (May 2010). "Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior". Nature Genetics. 42 (5): 448–53. PMC 3080600
 . PMID 20418888. doi:10.1038/ng.573.
. PMID 20418888. doi:10.1038/ng.573. - ↑ Schumann G, Coin LJ, Lourdusamy A, Charoen P, Berger KH, Stacey D, et al. (April 2011). "Genome-wide association and genetic functional studies identify autism susceptibility candidate 2 gene (AUTS2) in the regulation of alcohol consumption". Proceedings of the National Academy of Sciences of the United States of America. 108 (17): 7119–24. PMC 3084048
 . PMID 21471458. doi:10.1073/pnas.1017288108.
. PMID 21471458. doi:10.1073/pnas.1017288108. - 1 2 Okbay A, Baselmans BM, De Neve JE, Turley P, Nivard MG, Fontana MA, et al. (June 2016). "Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses". Nature Genetics. 48 (6): 624–33. PMID 27089181. doi:10.1038/ng.3552.
- ↑ Day FR, Helgason H, Chasman DI, Rose LM, Loh PR, Scott RA, Helgason A, Kong A, Masson G, Magnusson OT, Gudbjartsson D, Thorsteinsdottir U, Buring JE, Ridker PM, Sulem P, Stefansson K, Ong KK, Perry JR (June 2016). "Physical and neurobehavioral determinants of reproductive onset and success". Nature Genetics. 48 (6): 617–23. PMID 27089180. doi:10.1038/ng.3551.
- ↑ CONVERGE consortium (July 2015). "Sparse whole-genome sequencing identifies two loci for major depressive disorder". Nature. 523 (7562): 588–91. PMC 4522619
 . PMID 26176920. doi:10.1038/nature14659.
. PMID 26176920. doi:10.1038/nature14659. - ↑ Hibar DP, Stein JL, Renteria ME, Arias-Vasquez A, Desrivières S, Jahanshad N, et al. (April 2015). "Common genetic variants influence human subcortical brain structures". Nature. 520 (7546): 224–9. PMC 4393366
 . PMID 25607358. doi:10.1038/nature14101.
. PMID 25607358. doi:10.1038/nature14101. - ↑ Iacono WG, Vaidyanathan U, Vrieze SI, Malone SM (December 2014). "Knowns and unknowns for psychophysiological endophenotypes: integration and response to commentaries". Psychophysiology. 51 (12): 1339–47. PMC 4231488
 . PMID 25387720. doi:10.1111/psyp.12358.
. PMID 25387720. doi:10.1111/psyp.12358. - ↑ Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC, Rimmler JB, Locke PA, Conneally PM, Schmader KE (June 1994). "Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease". Nature Genetics. 7 (2): 180–4. PMID 7920638. doi:10.1038/ng0694-180.
- ↑ Luczak SE, Glatt SJ, Wall TL (July 2006). "Meta-analyses of ALDH2 and ADH1B with alcohol dependence in Asians". Psychological Bulletin. 132 (4): 607–21. PMID 16822169. doi:10.1037/0033-2909.132.4.607.
- ↑ Guerreiro RJ, Gustafson DR, Hardy J (March 2012). "The genetic architecture of Alzheimer's disease: beyond APP, PSENs and APOE". Neurobiology of Aging. 33 (3): 437–56. PMC 2980860
 . PMID 20594621. doi:10.1016/j.neurobiolaging.2010.03.025.
. PMID 20594621. doi:10.1016/j.neurobiolaging.2010.03.025. - ↑ Gusella JF, Wexler NS, Conneally PM, Naylor SL, Anderson MA, Tanzi RE, Watkins PC, Ottina K, Wallace MR, Sakaguchi AY (1983). "A polymorphic DNA marker genetically linked to Huntington's disease". Nature. 306 (5940): 234–8. PMID 6316146. doi:10.1038/306234a0.
Further reading
- Plomin R, DeFries JC, Knopik VS, Neiderhiser JM (January 2016). "Top 10 Replicated Findings From Behavioral Genetics". Perspectives on Psychological Science. 11 (1): 3–23. PMID 26817721. doi:10.1177/1745691615617439.
- Crusio WE (2015). "Key issues in contemporary behavioral genetics". Current Opinion in Behavioral Sciences. 2: 89–95. doi:10.1016/j.cobeha.2014.10.002.
- Crusio WE, Gerlai RT, eds. (1999). Handbook of Molecular-Genetic Techniques for Brain and Behavior Research. Techniques in the Behavioral and Neural Sciences. 13. Elsevier. ISBN 978-0-444-50239-1.
- Crusio WE, Sluyter F, Gerlai RT, Pietropaolo S, eds. (2013). Behavioral Genetics of the Mouse: Genetics of Behavioral Phenotypes. Cambridge Handbooks in Behavioral Genetics. 1. Cambridge University Press. ISBN 978-1-107-03481-5.
- Flint J, Greenspan RJ, Kendler KS (28 January 2010). How Genes Influence Behavior. Oxford University Press. ISBN 978-0-19-955990-9. Lay summary (20 November 2013).
- Johnson W, Turkheimer E, Gottesman II, Bouchard TJ (August 2010). "Beyond Heritability: Twin Studies in Behavioral Research". Current Directions in Psychological Science. 18 (4): 217–220. PMC 2899491
 . PMID 20625474. doi:10.1111/j.1467-8721.2009.01639.x.
. PMID 20625474. doi:10.1111/j.1467-8721.2009.01639.x. - Johnson W, Penke L, Spinath FM (2011). "Understanding Heritability: What it is and What it is Not" (PDF). European Journal of Personality. 25 (4): 287–294. ISSN 0890-2070. doi:10.1002/per.835. Retrieved 15 December 2013.
- Maxson SC (10 October 2012). "Chapter 1: Behavioral Genetics". In Weiner IB, Nelson RJ, Mizumori S. Handbook of Psychology (PDF). Volume 3: Behavioral Neuroscience. John Wiley & Sons. ISBN 978-0-470-89059-2. Archived from the original on 2013. Retrieved 15 December 2013.
- Panofsky A (2014). Misbehaving Science. Controversy and the Development of Behavior Genetics. Chicago: University of Chicago Press. ISBN 978-0-226-05831-3.
- Plomin R, DeFries JC, Knopik VS, Jenae M. Neiderhiser (24 September 2012). Behavioral Genetics. Shaun Purcell (Appendix: Statistical Methods in Behaviorial Genetics). Worth Publishers. ISBN 978-1-4292-4215-8. Retrieved 4 September 2013. Lay summary (4 September 2013).
- Spinath FM, Johnson W (2011). "Chapter 10: Behavior Genetics". In Chamorro-Premuzic T, von Stumm S, Furnham A. The Wiley-Blackwell Handbook of Individual Differences. United Kingdom: Blackwell Publishing Ltd. ISBN 978-1-4443-3438-8. doi:10.1002/9781444343120. Lay summary (10 July 2013).
External links
- McGue, Matt (5 May 2014). "Introduction to Human Behavioral Genetics". Coursera. Retrieved 10 June 2014. Free Massively Open Online Course on human behaviour genetics by Matt McGue.
- Institute for Behavioral Genetics at the University of Colorado Boulder
- Virginia Institute for Psychiatric and Behavioral Genetics
