Enterobacteria phage T4
| Enterobacteria phage T4 | |
|---|---|
 | |
| Virus classification | |
| Group: | Group I (dsDNA) |
| Order: | Caudovirales |
| Family: | Myoviridae |
| Subfamily: | Tevenvirinae |
| Genus: | T4virus |
| Species: | Enterobacteria phage T4 |
Enterobacteria phage T4 is a bacteriophage that infects Escherichia coli bacteria. The T4 phage is a member of the T-even phages, a group including enterobacteriophages T2 and T6. T4 is capable of undergoing only a lytic lifecycle and not the lysogenic lifecycle.
Genome and structure
The T4 phage's double-stranded DNA genome is about 169 kbp long[1] and encodes 289 proteins. The T4 genome is terminally redundant and is first replicated as a unit, then several genomic units are recombined end-to-end to form a concatemer. When packaged, the concatemer is cut at unspecific positions of the same length, leading to several genomes that represent circular permutations of the original.[2] The T4 genome bears eukaryote-like intron sequences.
Translation
The Shine-Dalgarno sequence GAGG dominates in bacteriophage T4 early genes, whereas the sequence GGAG is a target for the T4 endonuclease RegB that initiates the early mRNA degradation.[3]
Virus particle structure
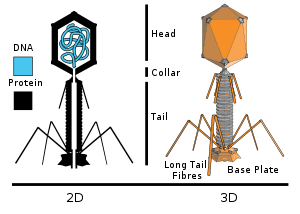
T4 is a relatively large phage, at approximately 90 nm wide and 200 nm long (most phages range from 25 to 200 nm in length). The DNA genome is held in an icosahedral head, also known as a capsid. The T4’s tail is hollow so that it can pass its nucleic acid into the cell it is infecting after attachment. The tail attaches to a host cell with the help of tail fibres. The tail fibres are also important in recognizing host cell surface receptors, so they determine if a bacterium is within the phage's host range.[4]
The structure of the 6 megadalton T4 baseplate that comprises 127 polypeptide chains of 13 different proteins (gene products 5, 5.4, 6, 7, 8, 9, 10, 11, 12, 25, 27, 48 and 53) has recently been described in atomic detail. An atomic model of the proximal region of the tail tube formed by gp54 and the main tube protein gp19 have also been created. The tape measure protein gp29 is present in the baseplate-tail tube complexes, but it could not be modeled.[5]
Infection process
The T4 phage initiates an Escherichia coli infection by binding OmpC porin proteins and Lipopolysaccharide (LPS) on the surface of E. coli cells with its long tail fibers (LTF).[6][7] A recognition signal is sent through the LTFs to the baseplate. This unravels the short tail fibers (STF) that bind irreversibly to the E. coli cell surface. The baseplate changes conformation and the tail sheath contracts, causing GP5 at the end of the tail tube to puncture the outer membrane of the cell. The lysozyme domain of GP5 is activated and degrades the periplasmic peptidoglycan layer. The remaining part of the membrane is degraded and then DNA from the head of the phage can travel through the tail tube and enter the E. coli cell.
Reproduction
The lytic lifecycle (from entering a bacterium to its destruction) takes approximately 30 minutes (at 37 °C) and consists of:
- Adsorption and penetration (starting immediately)
- Arrest of host gene expression (starting immediately)
- Enzyme synthesis (starting after 5 minutes)
- DNA replication (starting after 10 minutes)
- Formation of new virus particles (starting after 12 minutes)
After the life cycle is complete, the host cell bursts open and ejects the newly built viruses into the environment, destroying the host cell. T4 has a burst size of approximately 100-150 viral particles per infected host. Complementation, deletion, and recombination tests can be used to map out the rII gene locus by using T4. These bacteriophage infect a host cell with their information and then blow up the host cell, thereby propagating themselves.
Replication and packaging
The rate of DNA replication in a living cell was measured as the rate of phage T4 DNA elongation in phage-infected E. coli.[8] During the period of exponential DNA increase at 37 °C, the rate was 749 nucleotides per second. The mutation rate per base pair per replication during phage T4 DNA synthesis is 1.7 per 10−8,[9] a highly accurate DNA copying mechanism, with only 1 error in 300 copies. The phage also codes for unique DNA repair mechanisms. The T4 DNA packaging motor has been found to load DNA into phage capsids at a rate up to 2000 base pairs per second. The power involved, if scaled up in size, would be equivalent to that of an average automobile engine.[10]
Multiplicity reactivation
Multiplicity reactivation (MR) is the process by which two or more virus genomes, each containing inactivating genome damage, can interact within an infected cell to form a viable virus genome. Salvador Luria, while studying UV irradiated phage T4 in 1946, discovered MR and proposed that the observed reactivation of damaged phage occurs by a recombination mechanism.(see refs.[11][12][13]) This preceded the confirmation of DNA as the genetic material in 1952 in related phage T2 by the Hershey–Chase experiment.[14]
As remembered by Luria (1984,[15] pg. 97) the discovery of reactivation of irradiated phage (referred to as "multiplicity reactivation") immediately started a flurry of activity in the study of repair of radiation damage within the early phage group (reviewed by Bernstein[16] in 1981). It turned out later that the repair of damaged phage by mutual help that Luria had discovered was only one special case of DNA repair. Cells of all types, not just, bacteria and their viruses, but all organisms studied, including humans, are now known to have complex biochemical processes for repairing DNA damages (see DNA repair). DNA repair processes are also now recognized as playing critical roles in protecting against aging, cancer, and infertility.
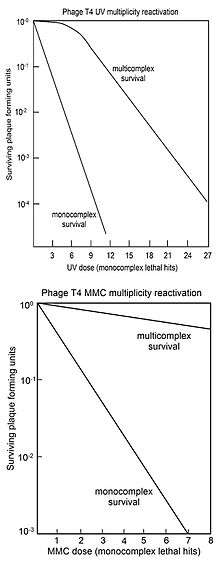
MR is usually represented by "survival curves" where survival of plaque forming ability of multiply infected cells (multicomplexes) is plotted against dose of genome damaging agent. For comparison, the survival of phage plaque forming ability of singly infected cells (monocomplexes) is also plotted against dose of genome damaging agent. The top figure shows the survival curves for phage T4 multicomplexes and monocomplexes with increasing dose of UV light. Since survival is plotted on a log scale it is clear that survival of multicomplexes exceeds that of monocomplexes by very large factors (depending on dose). The UV inactivation curve for multicomplexes has an initial shoulder. Other phage T4 DNA damaging agents with shoulders in their multicomplex survival curves are X-rays[17][18] and ethyl methane sulfonate (EMS).[16] The presence of a shoulder has been interpreted to mean that two recombinational processes are used.[19] The first one repairs DNA with high efficiency (in the "shoulder"), but is saturated in its ability as damage increases; the second pathway functions at all levels of damage. Surviving T4 phage released from multicomplexes show no increase in mutation, indicating that MR of UV irradiated phage is an accurate process.[19]
The bottom figure shows the survival curves for inactivation of phage T4 by the DNA damaging agent mitomycin C (MMC). In this case the survival curve for multicomplexes has no initial shoulder, suggesting that only the second recombinational repair process described above is active. The efficiency of repair by this process is indicated by the observation that a dose of MMC that allows survival of only 1 in 1,000 monocomplexes allows survival of about 70% of multicomplexes. Similar multicomplex survival curves (without shoulders) were also obtained for the DNA damaging agents P32 decay, psoralen plus near-UV irradiation (PUVA), N-methyl-N'-nitro-N-nitrosoguanidine (MNNG), methyl methane sulfonate (MMS) and nitrous acid.[16]
Several of the genes found to be necessary for MR in phage T4 proved to be orthologs for genes essential for recombination in prokaryotes, eukaryotes and archaea. This includes, for instance, T4 gene uvsX[20] which specifies a protein that has three-dimensional structural homology to RecA from Escherichia coli and the homologous protein RAD51 in eukaryotes and RadA in archaea. It has been suggested that the efficient and accurate recombinational repair of DNA damages during MR may be analogous to the recombinational repair process that occurs during meiosis in eukaryotes.[21]
History
The specific time and place of T4 phage isolation remains unclear, though they were likely found in sewage or fecal material. T4 and similar phages were described in a paper by Thomas F. Anderson, Max Delbrück, and Milislav Demerec in November 1944.[22]
A number of Nobel Prize winners worked with phage T4 or T4-like phages including Max Delbrück, Salvador Luria, Alfred Hershey, James D. Watson, and Francis Crick. Other important scientists who worked with phage T4 include Michael Rossmann, Seymour Benzer, Bruce Alberts, Gisela Mosig,[23] Richard Lenski, and James Bull.
See also
References
- ↑ Miller, ES; Kutter, E; Mosig, G; Arisaka, F; Kunisawa, T; Rüger, W (March 2003). "Bacteriophage T4 genome.". Microbiology and molecular biology reviews : MMBR. 67 (1): 86–156, table of contents. PMC 150520
 . PMID 12626685. doi:10.1128/MMBR.67.1.86-156.2003.
. PMID 12626685. doi:10.1128/MMBR.67.1.86-156.2003. - ↑ Madigan M, Martinko J, eds. (2006). Brock Biology of Microorganisms (11th ed.). Prentice Hall. ISBN 0-13-144329-1.
- ↑ Malys N (2012). "Shine-Dalgarno sequence of bacteriophage T4: GAGG prevails in early genes". Molecular Biology Reports. 39 (1): 33–9. PMID 21533668. doi:10.1007/s11033-011-0707-4.
- ↑ Ackermann, H.-W.; Krisch, H. M. (6 April 2014). "A catalogue of T4-type bacteriophages". Archives of Virology. 142 (12): 2329–2345. PMID 9672598. doi:10.1007/s007050050246.
- ↑ Taylor, Nicholas M. I.; Prokhorov, Nikolai S.; Guerrero-Ferreira, Ricardo C.; Shneider, Mikhail M.; Browning, Christopher; Goldie, Kenneth N.; Stahlberg, Henning; Leiman, Petr G. "Structure of the T4 baseplate and its function in triggering sheath contraction". Nature. 533 (7603): 346–352. PMID 27193680. doi:10.1038/nature17971.
- ↑ Yu, F.; Mizushima, S. (1982). "Roles of lipopolysaccharide and outer membrane protein OmpC of Escherichia coli K-12 in the receptor function for bacteriophage T4". Journal of Bacteriology. 151 (2): 718–722. PMC 220313
 . PMID 7047495.
. PMID 7047495. - ↑ Furukawa, H.; Mizushima, S. (1982). "Roles of cell surface components of Escherichia coli K-12 in bacteriophage T4 infection: Interaction of tail core with phospholipids". Journal of Bacteriology. 150 (2): 916–924. PMC 216445
 . PMID 7040345.
. PMID 7040345. - ↑ McCarthy D, Minner C, Bernstein H, Bernstein C (1976). "DNA elongation rates and growing point distributions of wild-type phage T4 and a DNA-delay amber mutant". J Mol Biol. 106 (4): 963–81. PMID 789903. doi:10.1016/0022-2836(76)90346-6.
- ↑ Drake JW (1970) The Molecular Basis of Mutation. Holden-Day, San Francisco ISBN 0816224501 ISBN 978-0816224500
- ↑ Rao, Venigalla B; Black, Lindsay W (1 January 2010). "Structure and assembly of bacteriophage T4 head". Virology Journal. 7 (1): 356. doi:10.1186/1743-422X-7-356.
- ↑ Luria SE (1947). "Reactivation of Irradiated Bacteriophage by Transfer of Self-Reproducing Units". Proc. Natl. Acad. Sci. U.S.A. 33 (9): 253–64. PMC 1079044
 . PMID 16588748. doi:10.1073/pnas.33.9.253.
. PMID 16588748. doi:10.1073/pnas.33.9.253. - ↑ LURIA SE, DULBECCO R (1948). "Lethal mutations, and inactivation of individual genetic determinants in bacteriophage". Genetics. 33 (6): 618. PMID 18100306.
- ↑ Luria SE, Dulbecco R (1949). "Genetic Recombinations Leading to Production of Active Bacteriophage from Ultraviolet Inactivated Bacteriophage Particles". Genetics. 34 (2): 93–125. PMC 1209443
 . PMID 17247312.
. PMID 17247312. - ↑ HERSHEY AD, CHASE M (1952). "Independent functions of viral protein and nucleic acid in growth of bacteriophage". J. Gen. Physiol. 36 (1): 39–56. PMC 2147348
 . PMID 12981234. doi:10.1085/jgp.36.1.39.
. PMID 12981234. doi:10.1085/jgp.36.1.39. - ↑ Salvador E. Luria. A Slot Machine, A Broken Test Tube: An Autobiography. Harper & Row, New York: 1984. Pp. 228. ISBN 0-06-015260-5 (USA and Canada)
- 1 2 3 Bernstein C (1981). "Deoxyribonucleic acid repair in bacteriophage". Microbiol. Rev. 45 (1): 72–98. PMC 281499
 . PMID 6261109.
. PMID 6261109. - ↑ WATSON JD (1952). "The properties of x-ray inactivated bacteriophage". J. Bacteriol. 63 (4): 473–85. PMC 169298
 . PMID 14938320.
. PMID 14938320. - ↑ HARM W (1958). "Multiplicity reactivation, marker rescue, and genetic recombination in phage T4 following x-ray inactivation". Virology. 5 (2): 337–61. PMID 13544109. doi:10.1016/0042-6822(58)90027-8.
- 1 2 Yarosh DB (1978). "UV-induced mutation in bacteriophage T4". J. Virol. 26 (2): 265–71. PMC 354064
 . PMID 660716.
. PMID 660716. - ↑ Story RM, Bishop DK, Kleckner N, Steitz TA (1993). "Structural relationship of bacterial RecA proteins to recombination proteins from bacteriophage T4 and yeast". Science. 259 (5103): 1892–6. PMID 8456313. doi:10.1126/science.8456313.
- ↑ Bernstein C (1979). "Why are babies young? Meiosis may prevent aging of the germ line". Perspect. Biol. Med. 22 (4): 539–44. PMID 573881. doi:10.1353/pbm.1979.0041.
- ↑ Abedon, ST (June 2000). "The murky origin of Snow White and her T-even dwarfs.". Genetics. 155 (2): 481–6. PMC 1461100
 . PMID 10835374.
. PMID 10835374. - ↑ Nossal, NG; Franklin, JL; Kutter, E; Drake, JW (November 2004). "Anecdotal, historical and critical commentaries on genetics. Gisela Mosig.". Genetics. 168 (3): 1097–104. PMC 1448779
 . PMID 15579671.
. PMID 15579671.
Further reading
- Leiman P.G.; Kanamaru S; Mesyanzhinov V.V.; Arisaka F.; Rossmann M.G. (2003). "Structure and morphogenesis of bacteriophage T4.". Cellulal and Molecular Life Sciences CMLS. 60 (11): 2356–2370. doi:10.1007/s00018-003-3072-1.
- Karam, J., Petrov, V., Nolan, J., Chin, D., Shatley, C., Krisch, H., and Letarov, A. The T4-like phages genome project. http://phage.bioc.tulane.edu/. (The T4-like phage full genomic sequence depository)
- Mosig, G., and F. Eiserling. 2006. T4 and related phages: structure and development, R. Calendar and S. T. Abedon (eds.), The Bacteriophages. Oxford University Press, Oxford. (Review of phage T4 biology) ISBN 0-19-514850-9
- Filee J. Tetart F.; Suttle C.A.; Krisch H.M. (2005). "Marine T4-type bacteriophages, a ubiquitous component of the dark matter of the biosphere". Proc. Natl. Acad. Sci. USA. 102 (35): 12471–6. PMC 1194919
 . PMID 16116082. doi:10.1073/pnas.0503404102. (Indication of prevalence and T4-like phages in the wild)
. PMID 16116082. doi:10.1073/pnas.0503404102. (Indication of prevalence and T4-like phages in the wild) - Chibani-Chennoufi S.; Canchaya C.; Bruttin A.; Brussow H. (2004). "Comparative genomics of the T4-Like Escherichia coli phage JS98: implications for the evolution of T4 phages". J. Bacteriol. 186 (24): 8276–86. PMC 532421
 . PMID 15576776. doi:10.1128/JB.186.24.8276-8286.2004. (Characterization of a T4-like phage)
. PMID 15576776. doi:10.1128/JB.186.24.8276-8286.2004. (Characterization of a T4-like phage) - Desplats C, Krisch HM (May 2003). "The diversity and evolution of the T4-type bacteriophages". Res. Microbiol. 154 (4): 259–67. PMID 12798230. doi:10.1016/S0923-2508(03)00069-X.
- Miller, E.S.; Kutter E.; Mosig G.; Arisaka F.; Kunisawa T.; Ruger W. (2003). "Bacteriophage T4 genome". Microbiol. Mol. Biol. Rev. 67 (1): 86–156. PMC 150520
 . PMID 12626685. doi:10.1128/MMBR.67.1.86-156.2003. (Review of phage T4, from the perspective of its genome)
. PMID 12626685. doi:10.1128/MMBR.67.1.86-156.2003. (Review of phage T4, from the perspective of its genome) - Desplats C.; Dez C.; Tetart F.; Eleaume H.; Krisch H.M. (2002). "Snapshot of the genome of the pseudo-T-even bacteriophage RB49". J. Bacteriol. 184 (10): 2789–2804. PMC 135041
 . PMID 11976309. doi:10.1128/JB.184.10.2789-2804.2002. (Overview of the RB49 genome, a T4-like phage)
. PMID 11976309. doi:10.1128/JB.184.10.2789-2804.2002. (Overview of the RB49 genome, a T4-like phage) - Malys N, Chang DY, Baumann RG, Xie D, Black LW (2002). "A bipartite bacteriophage T4 SOC and HOC randomized peptide display library: detection and analysis of phage T4 terminase (gp17) and late sigma factor (gp55) interaction". J Mol Biol. 319 (2): 289–304. PMID 12051907. doi:10.1016/S0022-2836(02)00298-X. (T4 phage application in biotechnology for studying protein interaction)
- Tétart F.; Desplats C.; Kutateladze M.; Monod C.; Ackermann H.-W.; Krisch H.M. (2001). "Phylogeny of the major head and tail genes of the wide-ranging T4-type bacteriophages". J. Bacteriol. 183 (1): 358–366. PMC 94885
 . PMID 11114936. doi:10.1128/JB.183.1.358-366.2001. (Indication of the prevalence of T4-type sequences in the wild)
. PMID 11114936. doi:10.1128/JB.183.1.358-366.2001. (Indication of the prevalence of T4-type sequences in the wild) - Abedon S.T. (2000). "The murky origin of Snow White and her T-even dwarfs". Genetics. 155 (2): 481–6. PMC 1461100
 . PMID 10835374. (Historical description of the isolation of the T4-like phages T2, T4, and T6)
. PMID 10835374. (Historical description of the isolation of the T4-like phages T2, T4, and T6) - Ackermann HW, Krisch HM (1997). "A catalogue of T4-type bacteriophages". Arch. Virol. 142 (12): 2329–45. PMID 9672598. doi:10.1007/s007050050246. Archived from the original on 2001-11-01. (Nearly complete list of then-known T4-like phages)
- Monod C, Repoila F, Kutateladze M, Tétart F, Krisch HM (March 1997). "The genome of the pseudo T-even bacteriophages, a diverse group that resembles T4". J. Mol. Biol. 267 (2): 237–49. PMID 9096222. doi:10.1006/jmbi.1996.0867. (Overview of various T4-like phages from the perspective of their genomes)
- Kutter E.; Gachechiladze K.; Poglazov A.; Marusich E.; Shneider M.; Aronsson P.; Napuli A.; Porter D.; Mesyanzhinov V. (1995). "Evolution of T4-related phages". Virus Genes. 11 (2–3): 285–297. PMID 8828153. doi:10.1007/BF01728666. (Comparison of the genomes of various T4-like phages)
- Karam, J. D. et al. 1994. Molecular Biology of Bacteriophage T4. ASM Press, Washington, DC. (The second T4 bible, go here, as well as Mosig and Eiserling, 2006, to begin to learn about the biology T4 phage) ISBN 1-55581-064-0
- Eddy, S. R. 1992. Introns in the T-Even Bacteriophages. Ph.D. thesis. University of Colorado at Boulder. (Chapter 3 provides overview of various T4-like phages as well as the isolation of then-new T4-like phages)
- Surdis, T.J "et al" Bacteriophage attachment methods specific to T4, analysis, Overview.
- Mathews, C. K., E. M. Kutter, G. Mosig, and P. B. Berget. 1983. Bacteriophage T4. American Society for Microbiology, Washington, DC. (The first T4 bible; not all information here is duplicated in Karam et al., 1994; see especially the introductory chapter by Doermann for a historical overview of the T4-like phages) ISBN 0-914826-56-5
- Russell, R. L. 1967. Speciation Among the T-Even Bacteriophages. Ph.D. thesis. California Institute of Technology. (Isolation of the RB series of T4-like phages)
- Malys N, Nivinskas R (2009). "Non-canonical RNA arrangement in T4-even phages: accommodated ribosome binding site at the gene 26-25 intercistronic junction". Mol Microbiol. 73 (6): 1115–1127. PMID 19708923. doi:10.1111/j.1365-2958.2009.06840.x. (rare type of translational regulation characterized in T4)
- Kay D.; Fildes P. (1962). "Hydroxymethylcytosine-containing and tryptophan-dependent bacteriophages isolated from city effluents". J. Gen. Microbiol. 27: 143–6. PMID 14454648. doi:10.1099/00221287-27-1-143. (T4-like phage isolation, including that of phage Ox2)